Properties of square-pyramidal alkyl-thiolate Fe(III) complexes, including an analogue of the unmodified form of nitrile hydratase
- PMID: 18989922
- PMCID: PMC2659597
- DOI: 10.1021/ic801704n
Properties of square-pyramidal alkyl-thiolate Fe(III) complexes, including an analogue of the unmodified form of nitrile hydratase
Abstract
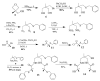
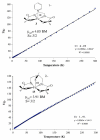
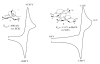
The syntheses and structures of three new coordinatively unsaturated, monomeric, square-pyramidal thiolate-ligated Fe(III) complexes are described, [Fe(III)((tame-N(3))S(2)(Me2))](+) (1), [Fe(III)(Et-N(2)S(2)(Me2))(py)](1-) (3), and [Fe(III)((tame-N(2)S)S(2)(Me2))](2-) (15). The anionic bis-carboxamide, tris-thiolate N(2)S(3) coordination sphere of 15 is potentially similar to that of the yet-to-be characterized unmodified form of NHase. Comparison of the magnetic and reactivity properties of these reveals how anionic charge build up (from cationic 1 to anionic 3 and dianionic 15) and spin-state influence apical ligand affinity. For all of the ligand-field combinations examined, an intermediate S = 3/2 spin state was shown to be favored by a strong N(2)S(2) basal plane ligand field, and this was found to reduce the affinity for apical ligands, even when they are built in. This is in contrast to the post-translationally modified NHase active site, which is low spin and displays a higher affinity for apical ligands. Cationic 1 and its reduced Fe(II) precursor are shown to bind NO and CO, respectively, to afford [Fe(III)((tame-N(3))S(2)(Me))(NO)](+) (18, nu(NuO) = 1865 cm(-1)), an analogue of NO-inactivated NHase, and [Fe(II)((tame-N(3))S(2)(Me))(CO)] (16; nu(CO) stretch (1895 cm(-1)). Anions (N(3)(-), CN(-)) are shown to be unreactive toward 1, 3, and 15 and neutral ligands unreactive toward 3 and 15, even when present in 100-fold excess and at low temperatures. The curtailed reactivity of 15, an analogue of the unmodified form of NHase, and its apical-oxygenated S = 3/2 derivative [Fe(III)((tame-N(2)SO(2))S(2)(Me2))](2-) (20) suggests that regioselective post-translational oxygenation of the basal plane NHase cysteinate sulfurs plays an important role in promoting substrate binding. This is supported by previously reported theoretical (DFT) calculations.
Figures



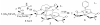




Similar articles
-
Use of metallopeptide based mimics demonstrates that the metalloprotein nitrile hydratase requires two oxidized cysteinates for catalytic activity.Inorg Chem. 2010 Oct 4;49(19):9064-77. doi: 10.1021/ic101765h. Inorg Chem. 2010. PMID: 20831172 Free PMC article.
-
A synthetic analogue of the active site of Fe-containing nitrile hydratase with carboxamido N and thiolato S as donors: synthesis, structure, and reactivities.J Am Chem Soc. 2001 Apr 11;123(14):3247-59. doi: 10.1021/ja001253v. J Am Chem Soc. 2001. PMID: 11457060
-
How does single oxygen atom addition affect the properties of an Fe-nitrile hydratase analogue? The compensatory role of the unmodified thiolate.J Am Chem Soc. 2006 Aug 30;128(34):11211-21. doi: 10.1021/ja062706k. J Am Chem Soc. 2006. PMID: 16925440 Free PMC article.
-
Fe(III) and Co(III) centers with carboxamido nitrogen and modified sulfur coordination: lessons learned from nitrile hydratase.Acc Chem Res. 2004 Apr;37(4):253-60. doi: 10.1021/ar0301532. Acc Chem Res. 2004. PMID: 15096062 Review.
-
Fe-type nitrile hydratase.J Inorg Biochem. 2001 Feb;83(4):247-53. doi: 10.1016/s0162-0134(00)00171-9. J Inorg Biochem. 2001. PMID: 11293544 Review.
Cited by
-
Increasing reactivity by incorporating π-acceptor ligands into coordinatively unsaturated thiolate-ligated iron(II) complexes.Inorganica Chim Acta. 2021 Sep 1;524:120422. doi: 10.1016/j.ica.2021.120422. Epub 2021 Apr 30. Inorganica Chim Acta. 2021. PMID: 34305163 Free PMC article.
-
Use of metallopeptide based mimics demonstrates that the metalloprotein nitrile hydratase requires two oxidized cysteinates for catalytic activity.Inorg Chem. 2010 Oct 4;49(19):9064-77. doi: 10.1021/ic101765h. Inorg Chem. 2010. PMID: 20831172 Free PMC article.
-
How Do Ring Size and π-Donating Thiolate Ligands Affect Redox-Active, α-Imino-N-heterocycle Ligand Activation?Inorg Chem. 2018 Feb 19;57(4):1935-1949. doi: 10.1021/acs.inorgchem.7b02748. Epub 2018 Feb 7. Inorg Chem. 2018. PMID: 29411979 Free PMC article.
-
Thioether-ligated iron(II) and iron(III)-hydroperoxo/alkylperoxo complexes with an H-bond donor in the second coordination sphere.Dalton Trans. 2014 May 28;43(20):7522-32. doi: 10.1039/c4dt00281d. Dalton Trans. 2014. PMID: 24705907 Free PMC article.
-
Sulfur versus iron oxidation in an iron-thiolate model complex.J Am Chem Soc. 2010 Dec 8;132(48):17118-29. doi: 10.1021/ja1045428. Epub 2010 Nov 11. J Am Chem Soc. 2010. PMID: 21070030 Free PMC article.
References
-
- Mitra S, Holz RC. J Biol Chem. 2007;282:7397–7404. - PubMed
-
- Noguchi T, Nojiri M, Takei K, Odaka M, Kamiya N. Biochemistry. 2003;42:11642–11650. - PubMed
-
- Kobayashi M, Shimizu S. Nature Biotechnology. 1998;16:733–736. - PubMed
-
- Sugiura Y, Kuwahara J, Nagasawa T, Yamada H. J. Am. Chem. Soc. 1987;109:5848–5850.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

