A simple microscale method for determining the relative stereochemistry of statine units
- PMID: 18989929
- PMCID: PMC2765571
- DOI: 10.1021/jo8012429
A simple microscale method for determining the relative stereochemistry of statine units
Erratum in
- J Org Chem. 2012 Apr 6;77(7):3686
Abstract
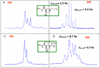
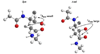
A simple method to determine the relative stereochemistry of statine amino acids (gamma-amino-beta-hydroxyacids) by using (1)H NMR spectroscopy is described. Configurational assignment of statine units within complex natural products is possible without degradation or derivatization as the syn and anti diastereomers can be distinguished by using a combination of chemical shift and coupling constant information derived from the alpha-methylene ABX system. Seventy-three examples are provided, demonstrating the scope and limitations of the methodology. These examples range in complexity from simple statine units to cyclic depsipeptides, such as tamandarin B.
Figures





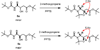
Similar articles
-
Unusual regioselection in the Mitsunobu reactions of syn-2,3-dihydroxy esters: synthesis of statine and its diastereomer.J Org Chem. 2002 Apr 19;67(8):2689-91. doi: 10.1021/jo015967f. J Org Chem. 2002. PMID: 11950319
-
Convenient synthetic route to an enantiomerically pure FMOC alpha-amino acid.J Org Chem. 2008 Dec 5;73(23):9334-9. doi: 10.1021/jo801781v. J Org Chem. 2008. PMID: 18986204
-
An NMR method for assigning relative stereochemistry to beta-hydroxy ketones deriving from aldol reactions of methyl ketones.J Org Chem. 2002 Jun 14;67(12):4284-9. doi: 10.1021/jo0164148. J Org Chem. 2002. PMID: 12054964
-
Asymmetric organocatalysis on a technical scale: current status and future challenges.Ernst Schering Found Symp Proc. 2007;(2):141-58. Ernst Schering Found Symp Proc. 2007. PMID: 18642524 Review. No abstract available.
-
An Nuclear Magnetic Resonance Fingerprint Matching Approach for the Identification and Structural Re-Evaluation of Pseudomonas Lipopeptides.Microbiol Spectr. 2022 Aug 31;10(4):e0126122. doi: 10.1128/spectrum.01261-22. Epub 2022 Jul 25. Microbiol Spectr. 2022. PMID: 35876524 Free PMC article. Review.
Cited by
-
Cytotoxic halogenated macrolides and modified peptides from the apratoxin-producing marine cyanobacterium Lyngbya bouillonii from Guam.J Nat Prod. 2010 Sep 24;73(9):1544-52. doi: 10.1021/np1004032. J Nat Prod. 2010. PMID: 20704304 Free PMC article.
-
Rational Design of Azastatin as a Potential ADC Payload with Reduced Bystander Killing.ChemMedChem. 2020 Dec 15;15(24):2500-2512. doi: 10.1002/cmdc.202000497. Epub 2020 Oct 16. ChemMedChem. 2020. PMID: 33063934 Free PMC article.
-
Thalassospiramide G, a new γ-amino-acid-bearing peptide from the marine bacterium Thalassospira sp.Mar Drugs. 2013 Feb 26;11(3):611-22. doi: 10.3390/md11030611. Mar Drugs. 2013. PMID: 23442790 Free PMC article.
-
Tasiamide F, a potent inhibitor of cathepsins D and E from a marine cyanobacterium.Bioorg Med Chem. 2016 Aug 1;24(15):3276-82. doi: 10.1016/j.bmc.2016.04.062. Epub 2016 Apr 30. Bioorg Med Chem. 2016. PMID: 27211244 Free PMC article.
-
Grassystatins A-C from marine cyanobacteria, potent cathepsin E inhibitors that reduce antigen presentation.J Med Chem. 2009 Sep 24;52(18):5732-47. doi: 10.1021/jm9009394. J Med Chem. 2009. PMID: 19715320 Free PMC article.
References
-
- Nicolaou KC, Snyder SA. Angew. Chem., Int. Ed. 2005;44:1012–1044. - PubMed
-
- Crews P, Rodriquez J, Jaspars M. Organic Structure Analysis. 1998
-
- Reynolds WF, Enriquez RG. J. Nat. Prod. 2002;65:221–244. - PubMed
-
- Matsumori N, Kaneno D, Murata M, Nakamura H, Tachibana K. J. Org. Chem. 1999;64:866–876. - PubMed
-
-
For a review on determining absolute configurations by NMR see: Seco JM, Quiñoá E, Riguera R. Chem. Rev. 2004;1:17–117..
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

