A single mutation in the nonamyloidogenic region of islet amyloid polypeptide greatly reduces toxicity
- PMID: 18989933
- PMCID: PMC2645932
- DOI: 10.1021/bi801427c
A single mutation in the nonamyloidogenic region of islet amyloid polypeptide greatly reduces toxicity
Abstract
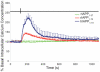
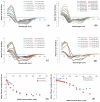
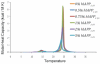
Islet amyloid polypeptide (IAPP or amylin) is a 37-residue peptide secreted with insulin by beta-cells in the islets of Langerhans. The aggregation of the peptide into either amyloid fibers or small soluble oligomers has been implicated in the death of beta-cells during type 2 diabetes through disruption of the cellular membrane. The actual form of the peptide responsible for beta-cell death has been a subject of controversy. Previous research has indicated that the N-terminal region of the peptide (residues 1-19) is primarily responsible for the membrane-disrupting effect of the hIAPP peptide and induces membrane disruption to a similar extent as the full-length peptide without forming amyloid fibers when bound to the membrane. The rat version of the peptide, which is both noncytotoxic and nonamyloidogenic, differs from the human peptide by only one amino acid residue: Arg18 in the rat version while His18 in the human version. To elucidate the effect of this difference, we have measured in this study the effects of the rat and human versions of IAPP(1-19) on islet cells and model membranes. Fluorescence microscopy shows a rapid increase in intracellular calcium levels of islet cells after the addition of hIAPP(1-19), indicating disruption of the cellular membrane, while the rat version of the IAPP(1-19) peptide is significantly less effective. Circular dichroism experiments and dye leakage assays on model liposomes show that rIAPP(1-19) is deficient in binding to and disrupting lipid membranes at low but not at high peptide to lipid ratios, indicating that the ability of rIAPP(1-19) to form small aggregates necessary for membrane binding and disruption is significantly less than hIAPP(1-19). At pH 6.0, where H18 is likely to be protonated, hIAPP(1-19) resembles rIAPP(1-19) in its ability to cause membrane disruption. Differential scanning calorimetry suggests a different mode of binding to the membrane for rIAPP(1-19) compared to hIAPP(1-19). Human IAPP(1-19) has a minimal effect on the phase transition of lipid vesicles, suggesting a membrane orientation of the peptide in which the mobility of the acyl chains of the membrane is relatively unaffected. Rat IAPP(1-19), however, has a strong effect on the phase transition of lipid vesicles at low concentrations, suggesting that the peptide does not easily insert into the membrane after binding to the surface. Our results indicate that the modulation of the peptide orientation in the membrane by His18 plays a key role in the toxicity of nonamyloidogenic forms of hIAPP.
Figures




References
-
- Clark A, Nilsson MR. Islet amyloid: a complication of islet dysfunction or an aetiological factor in Type 2 diabetes? Diabetologia. 2004;47:157–169. - PubMed
-
- Hoppener JWM, Lips CJM. Role of islet amyloid in type 2 diabetes mellitus. Int. J. Biochem. Cell Biol. 2006;38:726–736. - PubMed
-
- Lorenzo A, Yankner BA. Neurobiology of Alzheimer’s Disease. 1996. Amyloid fibril toxicity in Alzheimer’s disease and diabetes; pp. 89–95. - PubMed
-
- Matveyenko AV, Butler PC. Islet amyloid polypeptide (IAPP) transgenic rodents as models for type 2 diabetes. ILAR J. 2006;47:225–233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

