A small molecule inhibitor, 1,2,4,5-benzenetetraamine tetrahydrochloride, targeting the y397 site of focal adhesion kinase decreases tumor growth
- PMID: 18989950
- PMCID: PMC2662449
- DOI: 10.1021/jm800483v
A small molecule inhibitor, 1,2,4,5-benzenetetraamine tetrahydrochloride, targeting the y397 site of focal adhesion kinase decreases tumor growth
Abstract
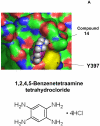
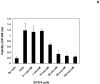
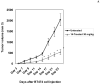
Focal adhesion kinase (FAK) is a nonreceptor kinase that is overexpressed in many types of tumors. We developed a novel cancer-therapy approach, targeting the main autophosphorylation site of FAK, Y397, by computer modeling and screening of the National Cancer Institute (NCI) small molecule compounds database. More than 140,000 small molecule compounds were docked into the N-terminal domain of the FAK crystal structure in 100 different orientations that identified 35 compounds. One compound, 14 (1,2,4,5-benzenetetraamine tetrahydrochloride), significantly decreased viability in most of the cells to the levels equal to or higher than control FAK inhibitor 1a (2-[5-chloro-2-[2-methoxy-4-(4-morpholinyl)phenylamino]pyrimidin-4-ylamino]-N-methylbenzamide, TAE226) from Novartis, Inc. Compound 14 specifically and directly blocked phosphorylation of Y397-FAK in a dose- and time-dependent manner. It increased cell detachment and inhibited cell adhesion in a dose-dependent manner. Furthermore, 14 effectively caused breast tumor regression in vivo. Thus, targeting the Y397 site of FAK with 14 inhibitor can be effectively used in cancer therapy.
Figures


















Similar articles
-
A novel small molecule inhibitor of FAK decreases growth of human pancreatic cancer.Cell Cycle. 2009 Aug;8(15):2435-43. doi: 10.4161/cc.8.15.9145. Epub 2009 Aug 1. Cell Cycle. 2009. PMID: 19571674 Free PMC article.
-
Focal adhesion kinase autophosphorylation inhibition decreases colon cancer cell growth and enhances the efficacy of chemotherapy.Cancer Biol Ther. 2013 Aug;14(8):761-72. doi: 10.4161/cbt.25185. Epub 2013 Jun 3. Cancer Biol Ther. 2013. PMID: 23792569 Free PMC article.
-
A small molecule focal adhesion kinase (FAK) inhibitor, targeting Y397 site: 1-(2-hydroxyethyl)-3, 5, 7-triaza-1-azoniatricyclo [3.3.1.1(3,7)]decane; bromide effectively inhibits FAK autophosphorylation activity and decreases cancer cell viability, clonogenicity and tumor growth in vivo.Carcinogenesis. 2012 May;33(5):1004-13. doi: 10.1093/carcin/bgs120. Epub 2012 Mar 7. Carcinogenesis. 2012. PMID: 22402131 Free PMC article.
-
Progress in the Development of Small Molecular Inhibitors of Focal Adhesion Kinase (FAK).J Med Chem. 2020 Dec 10;63(23):14382-14403. doi: 10.1021/acs.jmedchem.0c01248. Epub 2020 Oct 15. J Med Chem. 2020. PMID: 33058670 Review.
-
Focal adhesion kinase-An emerging viable target in cancer and development of focal adhesion kinase inhibitors.Chem Biol Drug Des. 2021 Mar;97(3):774-794. doi: 10.1111/cbdd.13808. Epub 2020 Nov 27. Chem Biol Drug Des. 2021. PMID: 33191630 Review.
Cited by
-
FAK is a critical regulator of neuroblastoma liver metastasis.Oncotarget. 2012 Dec;3(12):1576-87. doi: 10.18632/oncotarget.732. Oncotarget. 2012. PMID: 23211542 Free PMC article.
-
Kisspeptin-10 increases collagen content in the myocardium by focal adhesion kinase activity.Sci Rep. 2023 Nov 15;13(1):19977. doi: 10.1038/s41598-023-47224-3. Sci Rep. 2023. PMID: 37968564 Free PMC article.
-
Inhibition of cell-matrix adhesions prevents cartilage chondrocyte death following impact injury.J Orthop Res. 2014 Mar;32(3):448-54. doi: 10.1002/jor.22523. Epub 2013 Nov 19. J Orthop Res. 2014. PMID: 24249698 Free PMC article.
-
Nasal administration of recombinant osteopontin attenuates early brain injury after subarachnoid hemorrhage.Stroke. 2013 Nov;44(11):3189-94. doi: 10.1161/STROKEAHA.113.001574. Epub 2013 Sep 5. Stroke. 2013. PMID: 24008574 Free PMC article.
-
Understanding the roles of FAK in cancer: inhibitors, genetic models, and new insights.J Histochem Cytochem. 2015 Feb;63(2):114-28. doi: 10.1369/0022155414561498. Epub 2014 Nov 7. J Histochem Cytochem. 2015. PMID: 25380750 Free PMC article. Review.
References
-
- Schaller MD. The focal adhesion kinase. J Endocrinol. 1996;150(1):1–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

