Exploiting orthogonally reactive functionality: synthesis and stereochemical assignment of (-)-ushikulide A
- PMID: 18989964
- PMCID: PMC2655346
- DOI: 10.1021/ja807127s
Exploiting orthogonally reactive functionality: synthesis and stereochemical assignment of (-)-ushikulide A
Abstract
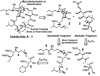
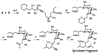
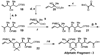
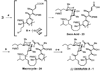
In spite of the tremendous advances in modern spectroscopic methods, organic synthesis continues to play a pivotal role in elucidating the full structures of complex natural products. This method has the advantage that, even in the absence of a firm structural assignment, a combination of logic and spectroscopic comparison can arrive at the correct structure. Herein, we report execution of this strategy with respect to ushikulide A, a newly isolated and previously stereochemically undefined member of the oligomycin-rutamycin family. To maximize synthetic efficiency, we envisioned chemoselective manipulation of orthogonally reactive functional groups, notably alkenes and alkynes as surrogates for certain carbonyl and hydroxyl functionalities. This approach has the dual effect of minimizing the number of steps and protecting groups required for our synthetic route. This strategy culminated in the efficient synthesis and stereochemical assignment of ushikulide A.
Figures


Similar articles
-
Total synthesis and stereochemical assignment of (-)-ushikulide A.J Am Chem Soc. 2009 Oct 21;131(41):15061-74. doi: 10.1021/ja906056v. J Am Chem Soc. 2009. PMID: 19775093 Free PMC article.
-
Interplay of cascade oxidative cyclization and hydride shifts in the synthesis of the ABC spiroketal ring system of pectenotoxin-4.Angew Chem Int Ed Engl. 2013 Feb 25;52(9):2491-4. doi: 10.1002/anie.201208919. Epub 2013 Jan 30. Angew Chem Int Ed Engl. 2013. PMID: 23362216
-
An approach toward constructing the trioxadispiroketal core in the DEF-ring of (+)-spirastrellolide A.Org Lett. 2014 Sep 5;16(17):4550-3. doi: 10.1021/ol502103b. Epub 2014 Aug 14. Org Lett. 2014. PMID: 25121803
-
Evolution of polyketides: post-PKS processing in the formation of spiroketals.Nat Prod Rep. 2008 Aug;25(4):651-5. doi: 10.1039/b719088n. Epub 2008 May 1. Nat Prod Rep. 2008. PMID: 18663388 Review.
-
Synthetic studies towards the pectenotoxins: a review.Org Biomol Chem. 2006 Nov 21;4(22):4048-58. doi: 10.1039/b611531b. Org Biomol Chem. 2006. PMID: 17312955 Review.
Cited by
-
Total synthesis and stereochemical assignment of (-)-ushikulide A.J Am Chem Soc. 2009 Oct 21;131(41):15061-74. doi: 10.1021/ja906056v. J Am Chem Soc. 2009. PMID: 19775093 Free PMC article.
-
The Enantioselective Addition of Alkyne Nucleophiles to Carbonyl Groups.Adv Synth Catal. 2009 May;351(7-8):10.1002/adsc.200800776. doi: 10.1002/adsc.200800776. Adv Synth Catal. 2009. PMID: 24353484 Free PMC article.
-
Thionium ion initiated medium-sized ring formation: the total synthesis of asteriscunolide D.J Am Chem Soc. 2012 Jan 25;134(3):1474-7. doi: 10.1021/ja210986f. Epub 2012 Jan 10. J Am Chem Soc. 2012. PMID: 22236456 Free PMC article.
-
Enantioselective ProPhenol-catalyzed addition of 1,3-diynes to aldehydes to generate synthetically versatile building blocks and diyne natural products.J Am Chem Soc. 2010 Apr 14;132(14):5186-92. doi: 10.1021/ja910656b. J Am Chem Soc. 2010. PMID: 20307084 Free PMC article.
-
Direct asymmetric Michael addition to nitroalkenes: vinylogous nucleophilicity under dinuclear zinc catalysis.J Am Chem Soc. 2009 Apr 8;131(13):4572-3. doi: 10.1021/ja809723u. J Am Chem Soc. 2009. PMID: 19281239 Free PMC article.
References
-
- Takahashi K, Yoshihara T, Kurosawa K. J. Antibiot. 2005;58:420. - PubMed
-
- Dreyfuss M, Harri E, Hoffmann H, Kobel H, Pache W, Tscherter H. Eur. J. Appl. Microbiol. 1976;3:125.
-
- Kino T, Hatanaka H, Hashimoto M, Nishiyama M, Goto T, Okuhara M, Kohsaka M, Aoki H, Imanaka H. J. Antibiot. 1987;40:1249. - PubMed
-
-
Attempts to prepare crystals of ushikulide A suitable for X-ray diffraction were unsuccessful in this case. Takahashi K. 2008 July 22; personal communication.
-
-
- Kirst HA, Mynderse JS, Martin JW, Baker PJ, Paschal JW, Steiner JL, Lobkovsky E, Clardy J. J. Antibiot. 1996;49:162. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

