Recruitment and enrollment for the simultaneous conduct of 2 randomized controlled trials for patients with subacute and chronic low back pain at a CAM research center
- PMID: 18990046
- PMCID: PMC3116528
- DOI: 10.1089/acm.2008.0066
Recruitment and enrollment for the simultaneous conduct of 2 randomized controlled trials for patients with subacute and chronic low back pain at a CAM research center
Abstract
Objective: To describe recruitment and enrollment experiences of 2 low back pain (LBP) randomized controlled trials (RCTs).
Design: Descriptive report.
Setting: Chiropractic research center in the midwest United States that is not a fee-for-service clinic.
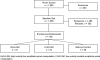
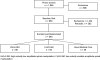
Participants: Both trials enrolled participants with subacute or chronic LBP without neurologic signs who had not received spinal manipulative care during the previous month. For study 1 we screened 1940 potential participants to enroll 192 participants (89 women and 103 men), mean age 40.0 +/- 9.4 years (range, 21-54 years). For study 2 we screened 1849 potential participants to enroll 240 participants (105 women and 135 men) at least 55 years old (mean, 63.1 +/- 6.7 years).
Interventions: Study 1 randomly assigned participants to 2 weeks of 2 different chiropractic techniques or a wait list control group. Study 2 randomly assigned participants to 6 weeks of 2 different chiropractic techniques or medical care consisting of 3 provider visits for medications.
Outcome measures: Recruitment source costs and yield, and baseline characteristics of enrolled versus nonparticipants were recorded.
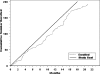
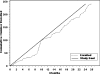
Results: We conducted 3789 telephone screens for both trials to enroll 432 (11%) participants, at a cost in excess of $156,000 for recruitment efforts. The cost per call for all callers averaged $41, ranging from $4 to $300 based on recruitment method; for enrolled participants, the cost per call was $361, ranging from $33 to $750. Direct mail efforts accounted for 62% of all callers, 57% for enrolled participants, and had the second lowest cost per call for recruitment efforts.
Conclusions: It is important that complementary and alternative medicine (CAM) research can be successfully conducted at CAM institutions. However, the costs associated with recruitment efforts for studies conducted at CAM institutions may be higher than expected and many self-identified participants are users of the CAM therapy. Therefore, strategies for efficient recruitment methods and targeting nonusers of CAM therapies should be developed early for CAM trials.
Trial registration: ClinicalTrials.gov NCT00285649 NCT00602901.
Figures
References
-
- Lovato LC. Hill K. Hertert S, et al. Recruitment for controlled clinical trials: Literature summary and annotated bibliography. Control Clin Trials. 1997;18:328–352. - PubMed
-
- Aitken L. Gallagher R. Madronio C. Principles of recruitment and retention in clinical trials. Int J Nurs Pract. 2003;9:338–346. - PubMed
-
- Oddone EZ. Olsen MK. Lindquist JH, et al. Enrollment in clinical trials according to patient's race: Experience from the VA Cooperative Studies Program (1975–2000) Control Clin Trials. 2004;25:378–387. - PubMed
-
- Cambron JA. Hawk C. Evans R, et al. Recruitment and accrual of women in a placebo-controlled clinical pilot study on manual therapy. J Manipulative Physiol Ther. 2004;27:299–305. - PubMed
-
- Cambron JA. Recruitment and accrual of women in a randomized controlled trial of spinal manipulation. J Manipulative Physiol Ther. 2001;24:79–83. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

