The transcriptomic signature of fasting murine liver
- PMID: 18990241
- PMCID: PMC2588605
- DOI: 10.1186/1471-2164-9-528
The transcriptomic signature of fasting murine liver
Abstract
Background: The contribution of individual organs to the whole-body adaptive response to fasting has not been established. Hence, gene-expression profiling, pathway, network and gene-set enrichment analysis and immunohistochemistry were carried out on mouse liver after 0, 12, 24 and 72 hours of fasting.
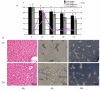
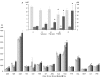
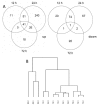
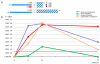
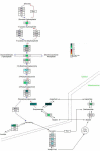
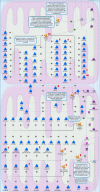
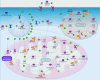
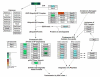
Results: Liver wet weight had declined approximately 44, approximately 5, approximately 11 and approximately 10% per day after 12, 24, 48 and 72 hours of fasting, respectively. Liver structure and metabolic zonation were preserved. Supervised hierarchical clustering showed separation between the fed, 12-24 h-fasted and 72 h-fasted conditions. Expression profiling and pathway analysis revealed that genes involved in amino-acid, lipid, carbohydrate and energy metabolism responded most significantly to fasting, that the response peaked at 24 hours, and had largely abated by 72 hours. The strong induction of the urea cycle, in combination with increased expression of enzymes of the tricarboxylic-acid cycle and oxidative phosphorylation, indicated a strong stimulation of amino-acid oxidation peaking at 24 hours. At this time point, fatty-acid oxidation and ketone-body formation were also induced. The induction of genes involved in the unfolded-protein response underscored the cell stress due to enhanced energy metabolism. The continuous high expression of enzymes of the urea cycle, malate-aspartate shuttle, and the gluconeogenic enzyme Pepck and the re-appearance of glycogen in the pericentral hepatocytes indicate that amino-acid oxidation yields to glucose and glycogen synthesis during prolonged fasting.
Conclusion: The changes in liver gene expression during fasting indicate that, in the mouse, energy production predominates during early fasting and that glucose production and glycogen synthesis become predominant during prolonged fasting.
Figures



References
-
- Le Maho Y, Vu Van Kha H, Koubi H, Dewasmes G, Girard J, Ferre P, Cagnard M. Body composition, energy expenditure, and plasma metabolites in long-term fasting geese. Am J Physiol. 1981;241:E342–E354. - PubMed
-
- Cherel Y, Attaix D, Rosolowska-Huszcz D, Belkhou R, Robin JP, Arnal M, Le MY. Whole-body and tissue protein synthesis during brief and prolonged fasting in the rat. Clin Sci (Lond) 1991;81:611–619. - PubMed
-
- Belkhou R, Cherel Y, Heitz A, Robin J, Le Maho Y. Energy contribution of proteins and lipids during prolonged fasting in the rat. Nutrition Research. 1991;11:365–374. doi: 10.1016/S0271-5317(05)80312-4. - DOI
-
- Habold C, Chevalier C, Dunel-Erb S, Foltzer-Jourdainne C, Le Maho Y, Lignot JH. Effects of fasting and refeeding on jejunal morphology and cellular activity in rats in relation to depletion of body stores. Scandinavian Journal of Gastroenterology. 2004;39:531–539. doi: 10.1080/00365520410004514. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

