A ligand-mediated nanovector for targeted gene delivery and transfection in cancer cells
- PMID: 18990439
- PMCID: PMC2709815
- DOI: 10.1016/j.biomaterials.2008.10.003
A ligand-mediated nanovector for targeted gene delivery and transfection in cancer cells
Abstract
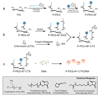
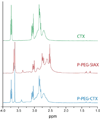
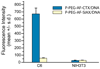
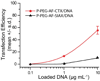
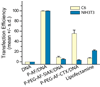
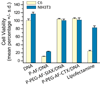
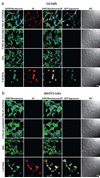
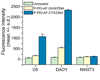
As conventional cancer therapies struggle with toxicity issues and irregular remedial efficacy, the preparation of novel gene therapy vectors could offer clinicians the tools for addressing the genetic errors of diseased tissue. The transfer of gene therapy to the clinic has proven difficult due to safety, target specificity, and transfection efficiency concerns. Polyethylenimine (PEI) nanoparticles have been identified as promising gene carriers that induce gene transfection with high efficiency. However, the inherent toxicity of the material and non-selective delivery are the major concerns in applying these particles clinically. Here, a non-viral nanovector has been developed by PEGylation of DNA-complexing PEI in nanoparticles functionalized with an Alexa Fluor 647 near infrared fluorophore, and the chlorotoxin (CTX) peptide which binds specifically to many forms of cancer. With this nanovector, the potential toxicity to healthy cells is minimized by both the reduction of the toxicity of PEI with the biocompatible copolymer and the targeted delivery of the nanovector to cancer cells, as evaluated by viability studies. The nanovector demonstrated high levels of targeting specificity and gene transfection efficiency with both C6 glioma and DAOY medulloblastoma tumor cells. Significantly, with the CTX as the targeting ligand, the nanovector may serve as a widely applicable gene delivery system for a broad array of cancer types.
Figures



References
-
- Stewart BW, Kleihues P. World Cancer Report. Geneva: World Health Organization Press; 2003.
-
- Ries L, Melbert D, Krapcho M, Stinchcomb DG, Howlader N, Horner MJ, et al. NCI, editor. SEER Cancer Statistics Review, 1975–2005. Bethesda, MD: 2007. SEER web site http://seer.cancer.gov/csr/1975_2005/
-
- Alton E, Ferrari S, Griesenbach U. Progress and prospects: Gene therapy clinical trials (part 2) Gene Ther. 2007;14(22):1555–1563. - PubMed
-
- Li SD, Huang L. Gene therapy progress and prospects: non-viral gene therapy by systemic delivery. Gene Ther. 2006;13(18):1313–1319. - PubMed
-
- McNeish IA, Bell SJ, Lemoine NR. Gene therapy progress and prospects: cancer gene therapy using tumour suppressor genes. Gene Ther. 2004;11(6):497–503. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

