Dietary soy supplement on fibromyalgia symptoms: a randomized, double-blind, placebo-controlled, early phase trial
- PMID: 18990724
- PMCID: PMC3136370
- DOI: 10.1093/ecam/nen069
Dietary soy supplement on fibromyalgia symptoms: a randomized, double-blind, placebo-controlled, early phase trial
Abstract
Most patients with fibromyalgia use complementary and alternative medicine (CAM). Properly designed controlled trials are necessary to assess the effectiveness of these practices. This study was a randomized, double-blind, placebo-controlled, early phase trial. Fifty patients seen at a fibromyalgia outpatient treatment program were randomly assigned to a daily soy or placebo (casein) shake. Outcome measures were scores of the Fibromyalgia Impact Questionnaire (FIQ) and the Center for Epidemiologic Studies Depression Scale (CES-D) at baseline and after 6 weeks of intervention. Analysis was with standard statistics based on the null hypothesis, and separation test for early phase CAM comparative trials. Twenty-eight patients completed the study. Use of standard statistics with intent-to-treat analysis showed that total FIQ scores decreased by 14% in the soy group (P = .02) and by 18% in the placebo group (P < .001). The difference in change in scores between the groups was not significant (P = .16). With the same analysis, CES-D scores decreased in the soy group by 16% (P = .004) and in the placebo group by 15% (P = .05). The change in scores was similar in the groups (P = .83). Results of statistical analysis using the separation test and intent-to-treat analysis revealed no benefit of soy compared with placebo. Shakes that contain soy and shakes that contain casein, when combined with a multidisciplinary fibromyalgia treatment program, provide a decrease in fibromyalgia symptoms. Separation between the effects of soy and casein (control) shakes did not favor the intervention. Therefore, large-sample studies using soy for patients with fibromyalgia are probably not indicated.
Figures
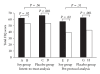

References
-
- Goldenberg DL, Burckhardt C, Crofford L. Management of fibromyalgia syndrome. Journal of the American Medical Association. 2004;292(19):2388–2395. - PubMed
-
- Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990. Criteria for the classification of fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis and Rheumatism. 1990;33(2):160–172. - PubMed
-
- Holdcraft LC, Assefi N, Buchwald D. Complementary and alternative medicine in fibromyalgia and related syndromes. Best Practice and Research: Clinical Rheumatology. 2003;17(4):667–683. - PubMed
-
- Rao JK, Mihaliak K, Kroenke K, Bradley J, Tierney WM, Weinberger M. Use of complementary therapies for arthritis among patients of rheumatologists. Annals of Internal Medicine. 1999;131(6):409–416. - PubMed
LinkOut - more resources
Full Text Sources
Medical

