Endothelial Src kinase regulates membrane recycling from the lateral border recycling compartment during leukocyte transendothelial migration
- PMID: 18991269
- PMCID: PMC2748968
- DOI: 10.1002/eji.200838605
Endothelial Src kinase regulates membrane recycling from the lateral border recycling compartment during leukocyte transendothelial migration
Abstract
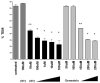
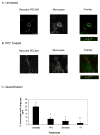
When leukocytes cross endothelial cells during the inflammatory response, membrane from the recently described lateral border recycling compartment (LBRC) is selectively targeted around diapedesing leukocytes. This "targeted recycling" is critical for leukocyte transendothelial migration. Blocking homophilic PECAM interactions between leukocytes and endothelial cells blocks targeted recycling from the LBRC and blocks diapedesis. However, the cellular signaling pathways that trigger targeted recycling are not known. We show that targeted recycling from the LBRC is dependent on Src kinase. The selective Src kinase inhibitor PP2 blocked targeted recycling and blocked diapedesis by over 70%. However, Src kinase inhibition did not affect the structure or normal constitutive recycling of membrane from the LBRC in the absence of leukocytes. PECAM, a Src kinase substrate, traffics between the LBRC and the endothelial surface at the cell border. However, virtually all of the PECAM in the cell that was phosphorylated on tyrosine residues was found in the LBRC. These findings demonstrate that Src kinase activity is critical for the targeted recycling of membrane from the LBRC to the site of transendothelial migration and that the PECAM in the LBRC is qualitatively different from the PECAM on the surface of endothelial cells.
Conflict of interest statement
Conflict of interest disclosure: The authors declare no competing financial interests.
Figures


References
-
- Muller WA. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends in Immunology. 2003;24:326–333. - PubMed
-
- Liao F, Schenkel AR, Muller WA. Transgenic mice expressing different levels of soluble platelet/endothelial cell adhesion molecule-IgG display distinct inflammatory phenotypes. J Immunol. 1999;163:5640–5648. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

