A review of coumarin derivatives in pharmacotherapy of breast cancer
- PMID: 18991629
- PMCID: PMC3772644
- DOI: 10.2174/092986708786242877
A review of coumarin derivatives in pharmacotherapy of breast cancer
Abstract
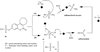
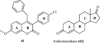
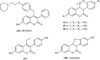
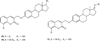
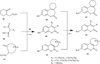
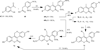
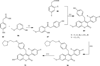
The coumarin (benzopyran-2-one, or chromen-2-one) ring system, present in natural products (such as the anticoagulant warfarin) that display interesting pharmacological properties, has intrigued chemists and medicinal chemists for decades to explore the natural coumarins or synthetic analogs for their applicability as drugs. Many molecules based on the coumarin ring system have been synthesized utilizing innovative synthetic techniques. The diversity oriented synthetic routes have led to interesting derivatives including the furanocoumarins, pyranocoumarins, and coumarin sulfamates (COUMATES), which have been found to be useful in photochemotherapy, antitumor and anti-HIV therapy, and as stimulants for central nervous system, antibacterials, anti-inflammatory, anti-coagulants, and dyes. Of particular interest in breast cancer chemotherapy, some coumarins and their active metabolite 7-hydroxycoumarin analogs have shown sulfatase and aromatase inhibitory activities. Coumarin based selective estrogen receptor modulators (SERMs) and coumarin-estrogen conjugates have also been described as potential antibreast cancer agents. Since breast cancer is the second leading cause of death in American women behind lung cancer, there is a strong impetus to identify potential new drug treatments for breast cancer. Therefore, the objective of this review is to focus on important coumarin analogs with antibreast cancer activities, highlight their mechanisms of action and structure-activity relationships on selected receptors in breast tissues, and the different methods that have been applied in the construction of these pharmacologically important coumarin analogs.
Figures
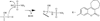







Similar articles
-
Medicinal chemistry aspects and synthetic strategies of coumarin as aromatase inhibitors: an overview.Med Oncol. 2022 Dec 5;40(1):41. doi: 10.1007/s12032-022-01916-4. Med Oncol. 2022. PMID: 36471176 Review.
-
Estrogen receptor agonists/antagonists in breast cancer therapy: A critical review.Bioorg Chem. 2017 Apr;71:257-274. doi: 10.1016/j.bioorg.2017.02.011. Epub 2017 Feb 23. Bioorg Chem. 2017. PMID: 28274582 Review.
-
A Review on Pharmacological Properties of Coumarins.Mini Rev Med Chem. 2018;18(2):113-141. doi: 10.2174/1389557516666160801094919. Mini Rev Med Chem. 2018. PMID: 27488585 Review.
-
Anti-breast Cancer Potential of Natural and Synthetic Coumarin Derivatives.Curr Top Med Chem. 2021;21(18):1692-1709. doi: 10.2174/1568026621666210303145430. Curr Top Med Chem. 2021. PMID: 33655862 Review.
-
Biochemical and biological characterization of a novel anti-aromatase coumarin derivative.J Biol Chem. 2004 Nov 12;279(46):48071-8. doi: 10.1074/jbc.M406847200. Epub 2004 Sep 8. J Biol Chem. 2004. PMID: 15358790
Cited by
-
The Effect of Furanocoumarin Derivatives on Induction of Apoptosis and Multidrug Resistance in Human Leukemic Cells.Molecules. 2019 May 12;24(9):1824. doi: 10.3390/molecules24091824. Molecules. 2019. PMID: 31083598 Free PMC article.
-
The Molecular and Structural Basis of O-methylation Reaction in Coumarin Biosynthesis in Peucedanum praeruptorum Dunn.Int J Mol Sci. 2019 Mar 27;20(7):1533. doi: 10.3390/ijms20071533. Int J Mol Sci. 2019. PMID: 30934718 Free PMC article.
-
One-pot synthesis, biological evaluation and molecular docking studies of fused thiazolo[2,3-b]pyrimidinone-pyrazolylcoumarin hybrids.Mol Divers. 2018 Nov;22(4):943-956. doi: 10.1007/s11030-018-9845-0. Epub 2018 Jul 2. Mol Divers. 2018. PMID: 29968120
-
Mutation at G103 of MtbFtsZ Altered their Sensitivity to Coumarins.Front Microbiol. 2017 Apr 6;8:578. doi: 10.3389/fmicb.2017.00578. eCollection 2017. Front Microbiol. 2017. PMID: 28428773 Free PMC article.
-
A novel coumarin derivative DBH2 inhibits proliferation and induces apoptosis of chronic myeloid leukemia cells.Genes Dis. 2022 Sep 8;10(2):596-607. doi: 10.1016/j.gendis.2022.08.021. eCollection 2023 Mar. Genes Dis. 2022. PMID: 37223541 Free PMC article.
References
-
- Egan D, O'Kennedy R, Moran E, Cox D, Prosser E, Thornes RD. Drug Metab. Rev. 1990;22:503. - PubMed
-
- Borges F, Roleira F, Milhazes N, Santana L, Uriarte E. Curr. Med. Chem. 2005;12:887. - PubMed
-
- Murray RDH, Mendez J, Brown SA. The Natural Coumarins: Occurrence, Chemistry and Biochemistry. New York: Wiley; 1982.
-
- Lacy A, O'Kennedy R. Curr. Pharm. Des. 2004;10:3797. - PubMed
-
- Gottlieb OR, Herrmann K, Murray RDH, Ohloff G, Pattenden G. Progress in the Chemistry of Organic Natural Products. New York: Springer-Verlag; 1978.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

