Novobiocin and additional inhibitors of the Hsp90 C-terminal nucleotide-binding pocket
- PMID: 18991631
- PMCID: PMC2729083
- DOI: 10.2174/092986708786242895
Novobiocin and additional inhibitors of the Hsp90 C-terminal nucleotide-binding pocket
Abstract
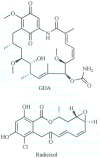
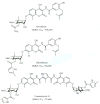
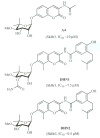
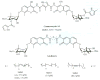
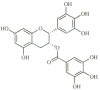
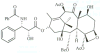
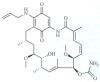
The 90 kDa heat shock proteins (Hsp90), which are integrally involved in cell signaling, proliferation, and survival, are ubiquitously expressed in cells. Many proteins in tumor cells are dependent upon the Hsp90 protein folding machinery for their stability, refolding, and maturation. Inhibition of Hsp90 uniquely targets client proteins associated with all six hallmarks of cancer. Thus, Hsp90 has emerged as a promising target for the treatment of cancer. Hsp90 exists as a homodimer, which contains three domains. The N-terminal domain contains an ATP-binding site that binds the natural products geldanamycin and radicicol. The middle domain is highly charged and has high affinity for co-chaperones and client proteins. Initial studies by Csermely and co-workers suggested a second ATP-binding site in the C-terminus of Hsp90. This C-terminal nucleotide binding pocket has been shown to not only bind ATP, but cisplatin, novobiocin, epilgallocatechin-3-gallate (EGCG) and taxol. The coumarin antibiotics novobiocin, clorobiocin, and coumermycin A1 were isolated from several streptomyces strains and exhibit potent activity against Gram-positive bacteria. These compounds bind type II topoisomerases, including DNA gyrase, and inhibit the enzyme-catalyzed hydrolysis of ATP. As a result, novobiocin analogues have garnered the attention of numerous researchers as an attractive agent for the treatment of bacterial infection. Novobiocin was reported to bind weakly to the newly discovered Hsp90 C-terminal ATP binding site ( approximately 700 M in SkBr3 cells) and induce degradation of Hsp90 client proteins. Structural modification of this compound has led to an increase of 1000-fold in activity in anti-proliferative assays. Recent studies of structure-activity relationship (SAR) by Renoir and co-workers highlighted the crucial role of the C-4 and/or C-7 positions of the coumarin and removal of the noviose moiety, which appeared to be essential for degradation of Hsp90 client proteins. Unlike the N-terminal ATP binding site, there is no reported co-crystal structure of Hsp90 C-terminus bound to any inhibitor. The Hsp90 C-terminal domain, however, is known to contain a conserved pentapeptide sequence (MEEVD) which is recognized by co-chaperones. Cisplatin is a platinum-containing chemotherapeutic used to treat various types of cancers, including testicular, ovarian, bladder, and small cell lung cancer. Most notably, cisplatin coordinates to DNA bases, resulting in cross-linked DNA, which prohibits rapidly dividing cells from duplicating DNA for mitosis. Itoh and co-workers reported that cisplatin decreases the chaperone activity of Hsp90. This group applied bovine brain cytosol to a cisplatin affinity column, eluted with cisplatin and detected Hsp90 in the eluent. Subsequent experiments indicated that cisplatin exhibits high affinity for Hsp90. Moreover Csermely and co-workers determined that the cisplatin binding site is located proximal to the C-terminal ATP binding site. EGCG is one of the active ingredients found in green tea. EGCG is known to inhibit the activity of many Hsp90-dependent client proteins, including telomerase, several kinases, and the aryl hydrocarbon receptor (AhR). Recently Gasiewicz and co-workers reported that EGCG manifests its antagonistic activity against AhR through binding Hsp90. Similar to novobiocin, EGCG was shown to bind the C-terminus of Hsp90. Unlike previously identified N-terminal Hsp90 inhibitors, EGCG does not appear to prevent Hsp90 from forming multiprotein complexes. Studies are currently underway to determine whether EGCG competes with novobiocin or cisplatin binding. Taxol, a well-known drug for the treatment of cancer, is responsible for the stabilization of microtubules and the inhibition of mitosis. Previous studies have shown that taxol induces the activation of kinases and transcription factors, and mimics the effect of bacterial lipopolysaccharide (LPS), an attribute unrelated to its tubulin-binding properties. Rosen and co-workers prepared a biotinylated taxol derivative and performed affinity chromatography experiments with lysates from both mouse brain and macrophage cell lines. These studies led to identification of two chaperones, Hsp70 and Hsp90, by mass spectrometry. In contrast to typical Hsp90-binding drugs, taxol exhibits a stimulatory response. Recently it was reported that the geldanamycin derivative 17-AAG behaves synergistically with taxol-induced apoptosis. This review describes the different C-terminal inhibitors of Hsp90, with specific emphasis on structure-activity relationship studies of novobiocin and their effects on anti-proliferative activity.
Figures






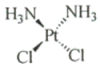
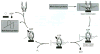
Similar articles
-
Novobiocin and related coumarins and depletion of heat shock protein 90-dependent signaling proteins.J Natl Cancer Inst. 2000 Feb 2;92(3):242-8. doi: 10.1093/jnci/92.3.242. J Natl Cancer Inst. 2000. PMID: 10655441
-
The heat shock protein 90 antagonist novobiocin interacts with a previously unrecognized ATP-binding domain in the carboxyl terminus of the chaperone.J Biol Chem. 2000 Nov 24;275(47):37181-6. doi: 10.1074/jbc.M003701200. J Biol Chem. 2000. PMID: 10945979
-
Novobiocin: redesigning a DNA gyrase inhibitor for selective inhibition of hsp90.J Am Chem Soc. 2006 Dec 6;128(48):15529-36. doi: 10.1021/ja065793p. J Am Chem Soc. 2006. PMID: 17132020
-
Novobiocin Analogs as Potential Anticancer Agents.Mini Rev Med Chem. 2017;17(9):728-733. doi: 10.2174/1389557516666161223155525. Mini Rev Med Chem. 2017. PMID: 28019639 Review.
-
Using natural product inhibitors to validate Hsp90 as a molecular target in cancer.Curr Top Med Chem. 2006;6(11):1163-71. doi: 10.2174/156802606777811979. Curr Top Med Chem. 2006. PMID: 16842153 Review.
Cited by
-
Heat shock protein 90 inhibition by 17-DMAG lessens disease in the MRL/lpr mouse model of systemic lupus erythematosus.Cell Mol Immunol. 2012 May;9(3):255-66. doi: 10.1038/cmi.2012.5. Epub 2012 Apr 30. Cell Mol Immunol. 2012. PMID: 22543833 Free PMC article.
-
Identification of Isoform-Selective Ligands for the Middle Domain of Heat Shock Protein 90 (Hsp90).Int J Mol Sci. 2019 Oct 26;20(21):5333. doi: 10.3390/ijms20215333. Int J Mol Sci. 2019. PMID: 31717777 Free PMC article.
-
Hsp90 in Human Diseases: Molecular Mechanisms to Therapeutic Approaches.Cells. 2022 Mar 12;11(6):976. doi: 10.3390/cells11060976. Cells. 2022. PMID: 35326427 Free PMC article. Review.
-
In Silico Discovery and Optimisation of a Novel Structural Class of Hsp90 C-Terminal Domain Inhibitors.Biomolecules. 2022 Jun 24;12(7):884. doi: 10.3390/biom12070884. Biomolecules. 2022. PMID: 35883440 Free PMC article.
-
Hsp90β inhibition modulates nitric oxide production and nitric oxide-induced apoptosis in human chondrocytes.BMC Musculoskelet Disord. 2011 Oct 17;12:237. doi: 10.1186/1471-2474-12-237. BMC Musculoskelet Disord. 2011. PMID: 22004293 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

