Imaging virus-associated cancer
- PMID: 18991718
- PMCID: PMC3369625
- DOI: 10.2174/138161208786404371
Imaging virus-associated cancer
Abstract
Cancer remains an important and growing health problem. Researchers have made great progress in defining genetic and molecular alterations that contribute to cancer formation and progression. Molecular imaging can identify appropriate patients for targeted cancer therapy and may detect early biochemical changes in tumors during therapy, some of which may have important prognostic implications. Progress in this field continues largely due to a union between molecular genetics and advanced imaging technology. This review details uses of molecular-genetic imaging in the context of tumor-associated viruses. Under certain conditions, and particularly during pharmacologic stimulation, gammaherpesviruses will express genes that enable imaging and therapy in vivo. The techniques discussed are readily translatable to the clinic.
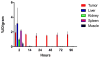
Figures


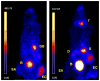
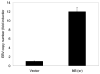
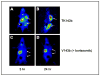


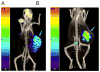


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

