Expression of BLIMP1/PRMT5 and concurrent histone H2A/H4 arginine 3 dimethylation in fetal germ cells, CIS/IGCNU and germ cell tumors
- PMID: 18992153
- PMCID: PMC2613889
- DOI: 10.1186/1471-213X-8-106
Expression of BLIMP1/PRMT5 and concurrent histone H2A/H4 arginine 3 dimethylation in fetal germ cells, CIS/IGCNU and germ cell tumors
Abstract
Background: Most testicular germ cell tumors arise from intratubular germ cell neoplasia unclassified (IGCNU, also referred to as carcinoma in situ), which is thought to originate from a transformed primordial germ cell (PGC)/gonocyte, the fetal germ cell. Analyses of the molecular profile of IGCNU and seminoma show similarities to the expression profile of fetal germ cells/gonocytes. In murine PGCs, expression and interaction of Blimp1 and Prmt5 results in arginine 3 dimethylation of histone H2A and H4. This imposes epigenetic modifications leading to transcriptional repression in mouse PGCs enabling them to escape the somatic differentiation program during migration, while expressing markers of pluripotency.
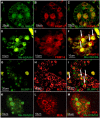
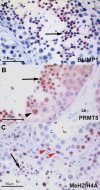
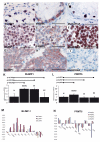
Results: In the present study, we show that BLIMP1 and PRMT5 were expressed and arginine dimethylation of histones H2A and H4 was detected in human male gonocytes at weeks 12-19 of gestation, indicating a role of this mechanism in human fetal germ cell development as well. Moreover, BLIMP1/PRMT5 and histone H2A and H4 arginine 3 dimethylation was present in IGCNU and most seminomas, while downregulated in embryonal carcinoma (EC) and other nonseminomatous tumors.
Conclusion: These data reveal similarities in marker expression and histone modification between murine and human PGCs. Moreover, we speculate that the histone H2A and H4 arginine 3 dimethylation might be the mechanism by which IGCNU and seminoma maintain the undifferentiated state while loss of these histone modifications leads to somatic differentiation observed in nonseminomatous tumors.
Figures


References
-
- Lee F, Hamid R, Arya M, Patel HR. Testicular cancer: current update and controversies. Hosp Med. 2002;63:615–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

