Liquid chromatography-mass spectrometry to study chondroitin lyase action pattern
- PMID: 18992215
- PMCID: PMC2636793
- DOI: 10.1016/j.ab.2008.10.014
Liquid chromatography-mass spectrometry to study chondroitin lyase action pattern
Abstract
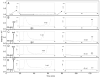
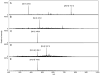
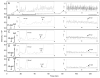
Liquid chromatography-mass spectrometry was applied to determine the action pattern of different chondroitin lyases. Two commercial enzymes, chondroitinase ABC (Proteus vulgaris) and chondroitinase ACII (Arthrobacter aurescens), having action patterns previously determined by viscosimetry and gel electrophoresis were first examined. Next, the action patterns of recombinant lyases, chondroitinase ABC from Bacteroides thetaiotaomicron (expressed in Escherichia coli) and chondroitinase AC from Flavobacterium heparinum (expressed in its original host), were examined. Chondroitin sulfate A (CS-A, also known as chondroitin-4-sulfate) was used as the substrate for these four lyases. Aliquots taken at various time points were analyzed. The products of chondroitinase ABC (P. vulgaris) and chondroitinase AC (F. heparinum) contained unsaturated oligosaccharides of sizes ranging from disaccharide to decasaccharide, demonstrating that both are endolytic enzymes. The products afforded by chondroitinase ABC (B. thetaiotaomicron) and chondroitinase ACII (A. aurescens) contained primarily unsaturated disaccharide. These two exolytic enzymes showed different minor products, suggesting some subtle specificity differences between the actions of these two exolytic lyases on chondroitin sulfate A.
Figures
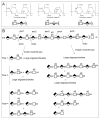
 as GlcA, and
as GlcA, and  as IdoA. The predominant sequence of CS-A, CS-B and CS-C are indicated. B. A single, typical chain of CS-A is shown with some sequence heterogeneity. The non-reducing end (nre) and reducing end (re) of the chain are indicated. An exolytic enzyme cuts the chain at each cleavage site starting from the nre while a random endolytic enzyme cuts the chain through the random selection of sites, typical product distribution at time points 1 (early reaction), 2 (intermediate reaction) and ∞ (reaction completion) are shown. The symbol
as IdoA. The predominant sequence of CS-A, CS-B and CS-C are indicated. B. A single, typical chain of CS-A is shown with some sequence heterogeneity. The non-reducing end (nre) and reducing end (re) of the chain are indicated. An exolytic enzyme cuts the chain at each cleavage site starting from the nre while a random endolytic enzyme cuts the chain through the random selection of sites, typical product distribution at time points 1 (early reaction), 2 (intermediate reaction) and ∞ (reaction completion) are shown. The symbol  corresponds to unsaturated uronic acid.
corresponds to unsaturated uronic acid.

Similar articles
-
Action pattern of polysaccharide lyases on glycosaminoglycans.Glycobiology. 1994 Jun;4(3):289-96. doi: 10.1093/glycob/4.3.289. Glycobiology. 1994. PMID: 7949654
-
Cloning and Expression of Recombinant Chondroitinase ACII and Its Comparison to the Arthrobacter aurescens Enzyme.Biotechnol J. 2017 Oct;12(10):10.1002/biot.201700239. doi: 10.1002/biot.201700239. Epub 2017 Sep 4. Biotechnol J. 2017. PMID: 28799715 Free PMC article.
-
Crystal structure of Proteus vulgaris chondroitin sulfate ABC lyase I at 1.9A resolution.J Mol Biol. 2003 May 2;328(3):623-34. doi: 10.1016/s0022-2836(03)00345-0. J Mol Biol. 2003. PMID: 12706721
-
Isolation and expression in Escherichia coli of cslA and cslB, genes coding for the chondroitin sulfate-degrading enzymes chondroitinase AC and chondroitinase B, respectively, from Flavobacterium heparinum.Appl Environ Microbiol. 2000 Jan;66(1):29-35. doi: 10.1128/AEM.66.1.29-35.2000. Appl Environ Microbiol. 2000. PMID: 10618199 Free PMC article.
-
The structures and applications of microbial chondroitin AC lyase.World J Microbiol Biotechnol. 2022 Aug 23;38(11):199. doi: 10.1007/s11274-022-03395-1. World J Microbiol Biotechnol. 2022. PMID: 35996038 Review.
Cited by
-
Exploiting enzyme specificities in digestions of chondroitin sulfates A and C: production of well-defined hexasaccharides.Glycobiology. 2012 Jun;22(6):826-38. doi: 10.1093/glycob/cws055. Epub 2012 Feb 17. Glycobiology. 2012. PMID: 22345629 Free PMC article.
-
Analysis of chondroitin degradation by components of a Bacteroides caccae polysaccharide utilization locus.J Biol Chem. 2025 Jul;301(7):110354. doi: 10.1016/j.jbc.2025.110354. Epub 2025 Jun 7. J Biol Chem. 2025. PMID: 40490139 Free PMC article.
-
Analysis of glycosaminoglycans in stem cell glycomics.Methods Mol Biol. 2011;690:285-300. doi: 10.1007/978-1-60761-962-8_19. Methods Mol Biol. 2011. PMID: 21043000 Free PMC article.
-
Glycosaminoglycans of the porcine central nervous system.Biochemistry. 2010 Nov 16;49(45):9839-47. doi: 10.1021/bi101305b. Epub 2010 Oct 26. Biochemistry. 2010. PMID: 20954748 Free PMC article.
-
High-performance liquid chromatography-mass spectrometry for mapping and sequencing glycosaminoglycan-derived oligosaccharides.Nat Protoc. 2010 Jun;5(6):993-1004. doi: 10.1038/nprot.2010.48. Nat Protoc. 2010. PMID: 20448545 Free PMC article.
References
-
- Volpi N. Chondroitin sulfate structure, role and pharmacological activity-advances in pharmacology. Academic Press; 2006. pp. 1–10.
-
- DeAngelis PL, Gunay NS, Toida T, Mao WJ, Linhardt RJ. Identification of the capsular polysaccharides of type D and F Pasteurella multocida as unmodified heparin and chondroitin, respectively. Carbohydr Res. 2002;337:1547–1552. - PubMed
-
- Bulow HE, Hobert O. The molecular diversity of glycosaminoglycans shapes animal development. Annu Rev Cell Dev Biol. 2006;22:375–407. - PubMed
-
- Linhardt RJ, Galliher PM, Cooney CL. Polysaccharide lyases. Appl Biochem Biotechnol. 1986;12:135–176. - PubMed
-
- Koketsu M, Linhardt RJ. Electrophoresis for the analysis of acidic oligosaccharides. Anal Biochem. 2000;283:136–145. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

