Ionic and neuromodulatory regulation of burst discharge controls frequency tuning
- PMID: 18992813
- PMCID: PMC4529324
- DOI: 10.1016/j.jphysparis.2008.10.019
Ionic and neuromodulatory regulation of burst discharge controls frequency tuning
Abstract
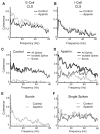
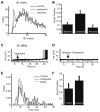
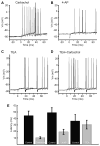
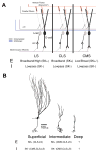
Sensory neurons encode natural stimuli by changes in firing rate or by generating specific firing patterns, such as bursts. Many neural computations rely on the fact that neurons can be tuned to specific stimulus frequencies. It is thus important to understand the mechanisms underlying frequency tuning. In the electrosensory system of the weakly electric fish, Apteronotus leptorhynchus, the primary processing of behaviourally relevant sensory signals occurs in pyramidal neurons of the electrosensory lateral line lobe (ELL). These cells encode low frequency prey stimuli with bursts of spikes and high frequency communication signals with single spikes. We describe here how bursting in pyramidal neurons can be regulated by intrinsic conductances in a cell subtype specific fashion across the sensory maps found within the ELL, thereby regulating their frequency tuning. Further, the neuromodulatory regulation of such conductances within individual cells and the consequences to frequency tuning are highlighted. Such alterations in the tuning of the pyramidal neurons may allow weakly electric fish to preferentially select for certain stimuli under various behaviourally relevant circumstances.
Figures


Similar articles
-
SK channels provide a novel mechanism for the control of frequency tuning in electrosensory neurons.J Neurosci. 2007 Aug 29;27(35):9491-502. doi: 10.1523/JNEUROSCI.1106-07.2007. J Neurosci. 2007. PMID: 17728462 Free PMC article.
-
Routing the flow of sensory signals using plastic responses to bursts and isolated spikes: experiment and theory.J Neurosci. 2011 Feb 16;31(7):2461-73. doi: 10.1523/JNEUROSCI.4672-10.2011. J Neurosci. 2011. PMID: 21325513 Free PMC article.
-
Oscillatory and burst discharge across electrosensory topographic maps.J Neurophysiol. 1996 Oct;76(4):2364-82. doi: 10.1152/jn.1996.76.4.2364. J Neurophysiol. 1996. PMID: 8899610
-
Neuromodulation of early electrosensory processing in gymnotiform weakly electric fish.J Exp Biol. 2013 Jul 1;216(Pt 13):2442-50. doi: 10.1242/jeb.082370. J Exp Biol. 2013. PMID: 23761469 Free PMC article. Review.
-
Feature extraction by burst-like spike patterns in multiple sensory maps.J Neurosci. 1998 Mar 15;18(6):2283-300. doi: 10.1523/JNEUROSCI.18-06-02283.1998. J Neurosci. 1998. PMID: 9482813 Free PMC article. Review.
Cited by
-
The central nervous system transcriptome of the weakly electric brown ghost knifefish (Apteronotus leptorhynchus): de novo assembly, annotation, and proteomics validation.BMC Genomics. 2015 Mar 11;16(1):166. doi: 10.1186/s12864-015-1354-2. BMC Genomics. 2015. PMID: 25879418 Free PMC article.
-
A fast BK-type KCa current acts as a postsynaptic modulator of temporal selectivity for communication signals.Front Cell Neurosci. 2014 Sep 17;8:286. doi: 10.3389/fncel.2014.00286. eCollection 2014. Front Cell Neurosci. 2014. PMID: 25278836 Free PMC article.
-
Distinct neuron phenotypes may serve object feature sensing in the electrosensory lobe of Gymnotus omarorum.J Exp Biol. 2021 May 1;224(9):jeb242242. doi: 10.1242/jeb.242242. Epub 2021 May 4. J Exp Biol. 2021. PMID: 33707195 Free PMC article.
-
Inhibition of SK and M channel-mediated currents by 5-HT enables parallel processing by bursts and isolated spikes.J Neurophysiol. 2011 Mar;105(3):1276-94. doi: 10.1152/jn.00792.2010. Epub 2011 Jan 5. J Neurophysiol. 2011. PMID: 21209357 Free PMC article.
-
Burst Firing in the Electrosensory System of Gymnotiform Weakly Electric Fish: Mechanisms and Functional Roles.Front Comput Neurosci. 2016 Aug 2;10:81. doi: 10.3389/fncom.2016.00081. eCollection 2016. Front Comput Neurosci. 2016. PMID: 27531978 Free PMC article. Review.
References
-
- Abbott LF. Where are the switches on this thing? In: van Hemmen JL, Sejnowski TJ, editors. 23 Problems in Systems Neuroscience. Oxford University Press; 2005. pp. 423–431.
-
- Andermann ML, Ritt J, Neimark MA, Moore CI. Neural correlates of vibrissa resonance; band-pass and somatotopic representation of high-frequency stimuli. Neuron. 2004;42:451–463. - PubMed
-
- Bastian J. Electrolocation II. The effects of moving objects and other electrical stimuli on the activities of two categories of posterior lateral line lobe cells in Apteronotus albifrons. J Comp Physiol [A] 1981;144:481–494.
-
- Bastian J. Plasticity of feedback inputs in the apteronotid electrosensory system. J Exp Biol. 1999;202(Pt 10):1327–1337. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

