Acyclic nucleoside bisphosphonates: synthesis and properties of chiral 2-amino-4,6-bis[(phosphonomethoxy)alkoxy]pyrimidines
- PMID: 18992968
- PMCID: PMC2706328
- DOI: 10.1016/j.ejmech.2008.09.031
Acyclic nucleoside bisphosphonates: synthesis and properties of chiral 2-amino-4,6-bis[(phosphonomethoxy)alkoxy]pyrimidines
Abstract
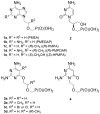
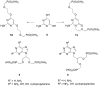
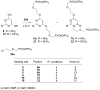
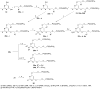
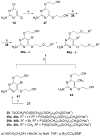
2-Amino-4,6-bis[(phosphonomethoxy)alkoxy]pyrimidines bearing two equal or different achiral or chiral phosphonoalkoxy chains have been prepared either by aromatic nucleophilic substitution of 2-amino-4,6-dichloropyrimidine or by alkylation of 4,6-dihydroxy-2-(methylsulfanyl)pyrimidine with appropriate phosphonate-bearing building block. Alkylation of 4,6-dihydroxy-2-(methylsulfanyl)pyrimidine proved to be the method of choice for efficient preparation of variety of bisphosphonates. The enantiomeric purity of selected compounds was determined by capillary electrophoresis. Antiviral activity of bisphosphonates is discussed.
Figures



References
-
- Naesens L, Snoeck R, Andrei G, Balzarini J, Neyts J, De Clercq E. Antivir Chem Chemother. 1997;8:1–23.
- Ying C, De Clercq E, Neyts J. J Viral Hepat. 2000;7:79–83. - PubMed
-
- Starrett JE, Jr, Tortolani DR, Hitchcock MJ, Martin JC, Mansuri MM. Antiviral Res. 1992;19:267–273. - PubMed
-
- Tsai CC, Follis KE, Beck TW, Sabo A, Bischofberger N, Dailey PJ. AIDS Res Hum Retroviruses. 1997;13:707–712. - PubMed
- Srinivas RV, Fridland A. Antimicrob Agents Chemother. 1998;42:1484–1487. - PMC - PubMed
- Suruga Y, Makino M, Okada Y, Tanaka H, De Clercq E, Baba M. J Acquired Immune Defic Syndr Hum Retrovirol. 1998;18:316–322. - PubMed
- Van Rompay KK, Miller MD, Marthas ML, Margot NA, Dailey PJ, Canfield DR, Tarara PR, Cherrington JM, Aguirre NL, Bischofberger N, Pedersen NC. J Virol. 2000;74:1767–1774. - PMC - PubMed
- Grim SA, Romanelli F. Annals of Pharmacother. 2003;37:849–859. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

