N-Cadherin and integrins: two receptor systems that mediate neuronal process outgrowth on astrocyte surfaces
- PMID: 18995807
- PMCID: PMC2623248
- DOI: 10.1016/j.neuron.2008.10.030
N-Cadherin and integrins: two receptor systems that mediate neuronal process outgrowth on astrocyte surfaces
Abstract
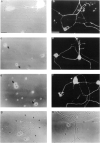
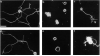
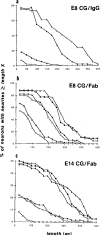
Receptor-mediated interactions between neurons and astroglia are likely to play a crucial role in the growth and guidance of CNS axons. Using antibodies to neuronal cell surface proteins, we identified two receptor systems mediating neurite outgrowth on cultured astrocytes. N-cadherin, a Ca2+-dependent cell adhesion molecule, functions prominently in the outgrowth of neurites on astrocytes by E8 and E14 chick ciliary ganglion (CC) neurons. β1-class integrin ECM receptor heterodimers function less prominently in E8 and not at all in E14 neurite outgrowth on astrocytes. The lack of effect of integrin β1 antibodies on E14 neurite outgrowth reflects an apparent loss of integrin function, as assayed by E14 neuronal attachment and process outgrowth on laminin. N-CAM appeared not to be required for neurite outgrowth by either E8 or E14 neurons. Since N-cadherin and integrin β1 antibodies together virtually eliminated E8 CG neurite outgrowth on cultured astrocytes, these two neuronal receptors are probably important in regulating axon growth on astroglia in vivo.
Figures

Comment on
-
N-cadherin and integrins: two receptor systems that mediate neuronal process outgrowth on astrocyte surfaces.Neuron. 1988 Mar;1(1):33-43. doi: 10.1016/0896-6273(88)90207-3. Neuron. 1988. PMID: 2856086
References
-
- Akers RM, Mosher DF, Lilien JE. Promotion of retinal neurite outgrowth by substratum-bound fibronectin. Dev. Biol. 1981;86:179–188. - PubMed
-
- Assouline JG, Bosch P, Lim R, Kim IS, Jensen R, Pantagis NJ. Rat astrocytes and Schwann cells in culture synthesize nerve growth factor-like neurite-promoting factors. Brain Res. 1987;428:103–118. - PubMed
-
- Benfey M, Aguayo AJ. Extensive elongation of axons from rat brain into peripheral nerve grafts. Nature. 1982;296:150–152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

