Hierarchical regulation of WASP/WAVE proteins
- PMID: 18995840
- PMCID: PMC2680354
- DOI: 10.1016/j.molcel.2008.10.012
Hierarchical regulation of WASP/WAVE proteins
Abstract
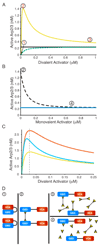
Members of the Wiskott-Aldrich syndrome protein (WASP) family control actin dynamics in eukaryotic cells by stimulating the actin nucleating activity of the Arp2/3 complex. The prevailing paradigm for WASP regulation invokes allosteric relief of autoinhibition by diverse upstream activators. Here we demonstrate an additional level of regulation that is superimposed upon allostery: dimerization increases the affinity of active WASP species for Arp2/3 complex by up to 180-fold, greatly enhancing actin assembly by this system. This finding explains a large and apparently disparate set of observations under a common mechanistic framework. These include WASP activation by the bacterial effector EspFu and a large number of SH3 domain proteins, the effects on WASP of membrane localization/clustering and assembly into large complexes, and cooperativity between different family members. Allostery and dimerization act in hierarchical fashion, enabling WASP/WAVE proteins to integrate different classes of inputs to produce a wide range of cellular actin responses.
Figures




References
-
- Banaszynski LA, Liu CW, Wandless TJ. Characterization of the FKBP.rapamycin.FRB ternary complex. J Am Chem Soc. 2005;127:4715–4721. - PubMed
-
- Bierne H, Miki H, Innocenti M, Scita G, Gertler FB, Takenawa T, Cossart P. WASP-related proteins, Abi1 and Ena/VASP are required for Listeria invasion induced by the Met receptor. J Cell Sci. 2005;118:1537–1547. - PubMed
-
- Campellone KG, Robbins D, Leong JM. EspFU is a translocated EHEC effector that interacts with Tir and N-WASP and promotes Nck-independent actin assembly. Dev Cell. 2004;7:217–228. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

