The SIRT2 deacetylase regulates autoacetylation of p300
- PMID: 18995842
- PMCID: PMC2645867
- DOI: 10.1016/j.molcel.2008.09.018
The SIRT2 deacetylase regulates autoacetylation of p300
Abstract
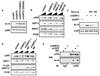
Autoacetylation of the p300 histone acetyltransferase controls the transition between VP16-mediated chromatin acetylation and preinitiation complex (PIC) assembly. Currently, it is unknown if and how autoacetylated p300 is deacetylated. We found that the NAD(+)-dependent histone deacetylase SIRT2 deacetylates p300 in vitro and in cells. SIRT2 deacetylates lysine residues in the catalytic domain of p300 and restores binding of p300 to the PIC. RNAi-mediated depletion or chemical inhibition of SIRT2 in cells results in accumulation of acetylated p300. The altered ac-p300/p300 ratio in SIRT2-depleted cells results in decreased p300 recruitment to an integrated VP16-responsive gene and inhibition of transcription. We conclude that p300 undergoes a dynamic cycle of autoacetylation and deacetylation.
Figures



References
-
- Arif M, Kumar GV, Narayana C, Kundu TK. Autoacetylation induced specific structural changes in histone acetyltransferase domain of p300: probed by surface enhanced Raman spectroscopy. J Phys Chem B. 2007;111:11877–11879. - PubMed
-
- Bergel M, Herrera JE, Thatcher BJ, Prymakowska-Bosak M, Vassilev A, Nakatani Y, Martin B, Bustin M. Acetylation of novel sites in the nucleosomal binding domain of chromosomal protein HMG-14 by p300 alters its interaction with nucleosomes. J Biol Chem. 2000;275:11514–11520. - PubMed
-
- Black JC, Choi JE, Lombardo SR, Carey M. A mechanism for coordinating chromatin modification and preinitiation complex assembly. Mol Cell. 2006;23:809–818. - PubMed
-
- Bouras T, Fu M, Sauve AA, Wang F, Quong AA, Perkins ND, Hay RT, Gu W, Pestell RG. SIRT1 deacetylation and repression of p300 involves lysine residues 1020/1024 within the cell cycle regulatory domain 1. J Biol Chem. 2005;280:10264–10276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

