Lysophosphatidic acid signaling in airway epithelium: role in airway inflammation and remodeling
- PMID: 18996473
- PMCID: PMC2660380
- DOI: 10.1016/j.cellsig.2008.10.010
Lysophosphatidic acid signaling in airway epithelium: role in airway inflammation and remodeling
Abstract
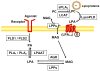
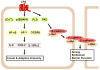
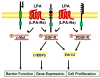
Lysophosphatidic acid (LPA), a potent bioactive phospholipid, induces diverse cellular responses, including cell proliferation, migration, and cytokine release. LPA can be generated intracellularly and extracellularly through multiple synthetic pathways by action of various enzymes, such as phospholipase A(1/2) (PLA(1/2)), phospholipase D (PLD), acylglycerol kinase (AGK), and lysophospholipase D (lysoPLD). Metabolism of LPA is regulated by a family of lipid phosphate phosphatases (LPPs). Significant amounts of LPA have been detected in various biological fluids, including serum, saliva, and bronchoalveolar lavage fluid (BALF). The most significant effects of LPA appear to be through activation of the G-protein-coupled receptors (GPCRs), termed LPA(1-6). LPA regulates gene expression through activation of several transcriptional factors, such as nuclear factor-kappaB (NF-kappaB), AP-1, and C/EBPbeta. In addition to GPCRs, cross-talk between LPA receptors and receptor tyrosine kinases (RTKs) partly regulates LPA-induced intracellular signaling and cellular responses. Airway epithelial cells participate in innate immunity through the release of cytokines, chemokines, lipid mediators, other inflammatory mediators and an increase in barrier function in response to a variety of inhaled stimuli. Expression of LPA receptors has been demonstrated in airway epithelial cells. This review summarizes our recent observations of the role of LPA/LPA-Rs in regulation of airway epithelium, especially in relation to the secretion of pro- and anti-inflammatory mediators and regulation of airway barrier function.
Figures
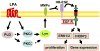
References
-
- Tigyi G, Miledi R. J Biol Chem. 1992;267(30):21360–21367. - PubMed
-
- Goetzl EJ, Lee H, Azuma T, Stossel TP, Turck CW, Karliner JS. J Biol Chem. 2000;275(19):14573–14578. - PubMed
-
- Hosogaya S, Yatomi Y, Nakamura K, Ohkawa R, Okubo S, Yokota H, Ohta M, Yamazaki H, Koike T, Ozaki Y. Ann Clin Biochem. 2008;45(Pt 4):364–368. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

