HDX-ESI-MS reveals enhanced conformational dynamics of the amyloidogenic protein beta(2)-microglobulin upon release from the MHC-1
- PMID: 18996721
- PMCID: PMC2642988
- DOI: 10.1016/j.jasms.2008.10.005
HDX-ESI-MS reveals enhanced conformational dynamics of the amyloidogenic protein beta(2)-microglobulin upon release from the MHC-1
Abstract
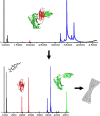
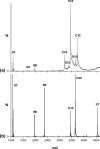
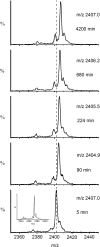
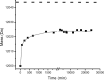
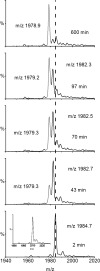
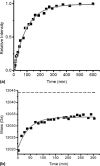
The light chain of the major histocompatibility complex class 1 (MHC-1), the protein beta(2)-microglobulin (beta(2)m), has amyloidogenic properties that arise only upon its dissociation from the MHC-1. Here hydrogen/deuterium exchange electrospray ionization mass spectrometry (HDX-ESI-MS) has been used to compare the solution dynamics of beta(2)m in its MHC-1 bound state compared with those of beta(2)m as a free monomer. The capability of tandem mass spectrometry to dissociate the MHC-1 into its individual constituents in the gas phase following deuterium incorporation in solution has permitted the direct observation of the exchange properties of MHC-1 bound beta(2)m for the first time. The HDX-ESI-MS data show clearly that the H-->D exchange of MHC-1 bound beta(2)m follows EX2 kinetics and that about 20 protons remain protected from exchange after 17 days. Free from the MHC-1, monomeric beta(2)m exhibits significantly different HDX behavior, which encompasses both EX1 and EX2 kinetics. The EX2 kinetics indicate a tenfold increase in the rate of exchange compared with MHC-1 bound beta(2)m, with just 10 protons remaining protected from EX2 exchange and therefore exchanging only via the EX1 mechanism. The EX1 kinetics observed for unbound beta(2)m are consistent with unfolding of its exchange-protected core with a t(1/2) of 68 min (pH 7, 37 degrees C). Thus, upon dissociation from the stabilizing influence of the MHC-1, free beta(2)m becomes highly dynamic and undergoes unfolding transitions that result in an aggregation-competent protein.
Figures
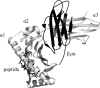
Similar articles
-
The role of conformational flexibility in β2-microglobulin amyloid fibril formation at neutral pH.Rapid Commun Mass Spectrom. 2012 Aug 30;26(16):1783-92. doi: 10.1002/rcm.6282. Rapid Commun Mass Spectrom. 2012. PMID: 22777780 Free PMC article.
-
Pulsed hydrogen/deuterium exchange MS/MS for studying the relationship between noncovalent protein complexes in solution and in the gas phase after electrospray ionization.Anal Chem. 2006 Mar 1;78(5):1613-9. doi: 10.1021/ac051687e. Anal Chem. 2006. PMID: 16503614
-
Protein structural dynamics at the gas/water interface examined by hydrogen exchange mass spectrometry.Protein Sci. 2015 Aug;24(8):1247-56. doi: 10.1002/pro.2680. Epub 2015 Apr 2. Protein Sci. 2015. PMID: 25761782 Free PMC article.
-
Protein structure and dynamics studied by mass spectrometry: H/D exchange, hydroxyl radical labeling, and related approaches.J Mass Spectrom. 2008 Aug;43(8):1021-36. doi: 10.1002/jms.1435. J Mass Spectrom. 2008. PMID: 18523973 Review.
-
Characterizing rapid, activity-linked conformational transitions in proteins via sub-second hydrogen deuterium exchange mass spectrometry.FEBS J. 2013 Nov;280(22):5616-25. doi: 10.1111/febs.12332. Epub 2013 Jun 11. FEBS J. 2013. PMID: 23663649 Review.
Cited by
-
Insights into the role of the beta-2 microglobulin D-strand in amyloid propensity revealed by mass spectrometry.Mol Biosyst. 2014 Mar 4;10(3):412-20. doi: 10.1039/c3mb70420c. Epub 2013 Dec 12. Mol Biosyst. 2014. PMID: 24336936 Free PMC article.
-
Comparing Hydrogen Deuterium Exchange and Fast Photochemical Oxidation of Proteins: a Structural Characterisation of Wild-Type and ΔN6 β2-Microglobulin.J Am Soc Mass Spectrom. 2018 Dec;29(12):2413-2426. doi: 10.1007/s13361-018-2067-y. Epub 2018 Sep 28. J Am Soc Mass Spectrom. 2018. PMID: 30267362 Free PMC article.
-
The role of conformational flexibility in β2-microglobulin amyloid fibril formation at neutral pH.Rapid Commun Mass Spectrom. 2012 Aug 30;26(16):1783-92. doi: 10.1002/rcm.6282. Rapid Commun Mass Spectrom. 2012. PMID: 22777780 Free PMC article.
-
Fast photochemical oxidation of proteins (FPOP): A powerful mass spectrometry-based structural proteomics tool.J Biol Chem. 2019 Aug 9;294(32):11969-11979. doi: 10.1074/jbc.REV119.006218. Epub 2019 Jul 1. J Biol Chem. 2019. PMID: 31262727 Free PMC article. Review.
-
Collagen I Weakly Interacts with the β-Sheets of β2-Microglobulin and Enhances Conformational Exchange To Induce Amyloid Formation.J Am Chem Soc. 2020 Jan 22;142(3):1321-1331. doi: 10.1021/jacs.9b10421. Epub 2020 Jan 8. J Am Chem Soc. 2020. PMID: 31875390 Free PMC article.
References
-
- MHC Sequencing Consortium Complete Sequence and Gene Map of a Human Major Histocompatibility Complex. Nature. 1999;401:921–923. - PubMed
-
- Madden D.R. The Three-Dimensional Structure of Peptide–MHC Complexes. Annu. Rev. Immunol. 1995;13:587–622. - PubMed
-
- Bjorkman P.J., Saper M.A., Samraoui B., Bennett W.S., Strominger J.L., Wiley D.C. Structure of the Human Class I Histocompatibility Antigen, HLA-A2. J. Immunol. 2005;174:6–12. - PubMed
-
- Jones E.Y., Tormo J., Reid S.W., Stuart D.I. Recognition Surfaces of MHC Class I. Immunol. Rev. 1998;163:121–128. - PubMed
-
- Boehm T., Zufall F. MHC Peptides and the Sensory Evaluation of Genotype. Trends Neurosci. 2006;29:100–107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

