Use of modified cornstarch therapy to extend fasting in glycogen storage disease types Ia and Ib
- PMID: 18996862
- PMCID: PMC3808112
- DOI: 10.3945/ajcn.2008.26352
Use of modified cornstarch therapy to extend fasting in glycogen storage disease types Ia and Ib
Abstract
Background: Type I glycogen storage disease (GSD) is caused by a deficiency of glucose-6-phosphatase resulting in severe fasting hypoglycemia.
Objective: We compared the efficacy of a new modified starch with the currently used cornstarch therapy in patients with type Ia and Ib GSD.
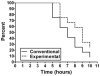
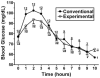
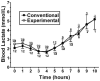
Design: This was a randomized, 2-d, double-blinded, crossover pilot study comparing the commonly used uncooked cornstarch with the experimental starch in 12 subjects (6 GSDIa, 6 GSDIb) aged >or=13 y. At 2200, the subjects were given 100 g of digestible starch, and glucose and lactate were measured hourly until the subject's plasma glucose concentration reached 60 mg/dL or until the subject had fasted for 10 h. The order in which the products were tested was randomized in a blinded fashion.
Results: The matched-pair Gehan rank test for censored survival was used to compare the therapies. The experimental starch maintained blood glucose concentrations significantly longer than did the traditional therapy (P = 0.013) in the 2-sided analysis. Most of the benefit was found to be after glucose concentrations fell below 70 mg/dL. The currently used cornstarch resulted in higher peak glucose concentrations and a more rapid rate of fall than did the new starch.
Conclusions: The experimental starch was superior to standard therapy in preventing hypoglycemia (<or=60 mg/dL). This therapy may allow patients with GSD to sleep through the night without awakening for therapy while enhancing safety. Additional studies are warranted to determine whether alternative dosing will further improve control in the therapeutic blood glucose range.
Figures
Similar articles
-
Cornstarch requirements of the adult glycogen storage disease Ia population: A retrospective review.J Inherit Metab Dis. 2020 Mar;43(2):269-278. doi: 10.1002/jimd.12160. Epub 2019 Sep 4. J Inherit Metab Dis. 2020. PMID: 31415093
-
A pilot longitudinal study of the use of waxy maize heat modified starch in the treatment of adults with glycogen storage disease type I: a randomized double-blind cross-over study.Orphanet J Rare Dis. 2015 Feb 15;10:18. doi: 10.1186/s13023-015-0229-6. Orphanet J Rare Dis. 2015. PMID: 25758258 Free PMC article. Clinical Trial.
-
Effect of continuous glucose therapy begun in infancy on the long-term clinical course of patients with type I glycogen storage disease.J Pediatr Gastroenterol Nutr. 1999 Aug;29(2):136-43. doi: 10.1097/00005176-199908000-00008. J Pediatr Gastroenterol Nutr. 1999. PMID: 10435649
-
Nutrition therapy for hepatic glycogen storage diseases.J Am Diet Assoc. 1993 Dec;93(12):1423-30. doi: 10.1016/0002-8223(93)92246-t. J Am Diet Assoc. 1993. PMID: 8245377 Review.
-
Glycogen Storage Disease Type Ia: Current Management Options, Burden and Unmet Needs.Nutrients. 2021 Oct 27;13(11):3828. doi: 10.3390/nu13113828. Nutrients. 2021. PMID: 34836082 Free PMC article. Review.
Cited by
-
Gene therapy and genome editing for type I glycogen storage diseases.Front Mol Med. 2023 Mar 31;3:1167091. doi: 10.3389/fmmed.2023.1167091. eCollection 2023. Front Mol Med. 2023. PMID: 39086673 Free PMC article. Review.
-
Hepatic glycogen storage diseases: pathogenesis, clinical symptoms and therapeutic management.Arch Med Sci. 2019 Feb 18;17(2):304-313. doi: 10.5114/aoms.2019.83063. eCollection 2021. Arch Med Sci. 2019. PMID: 33747265 Free PMC article.
-
Impact of hematopoietic stem cell transplantation in glycogen storage disease type Ib: A single-subject research design using 13C-glucose breath test.Mol Genet Metab Rep. 2023 Jan 3;34:100955. doi: 10.1016/j.ymgmr.2023.100955. eCollection 2023 Mar. Mol Genet Metab Rep. 2023. PMID: 36632325 Free PMC article.
-
Use of waxy maize heat modified starch in the treatment of children between 2 and 5 years with glycogen storage disease type I: A retrospective study.Mol Genet Metab Rep. 2019 Nov 6;21:100536. doi: 10.1016/j.ymgmr.2019.100536. eCollection 2019 Dec. Mol Genet Metab Rep. 2019. PMID: 31844626 Free PMC article.
-
mRNA therapy restores euglycemia and prevents liver tumors in murine model of glycogen storage disease.Nat Commun. 2021 May 25;12(1):3090. doi: 10.1038/s41467-021-23318-2. Nat Commun. 2021. PMID: 34035281 Free PMC article.
References
-
- Chen YT, Burchell A. Glycogen storage diseases. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic basis of inherited disease. 8th. New York, NY: McGraw-Hill; 2001.
-
- Lei KJ, Shelly LL, Pan CJ, et al. Mutations in the glucose-6-phosphatase gene that cause glycogen storage disease type Ia. Science. 1993;262:580–3. - PubMed
-
- Wolfsdorf JI, Holm IA, Weinstein DA. Glycogen storage diseases Phenotypic, genetic, and biochemical characteristics, and therapy. Endocrinol Metab Clin North Am. 1999;28:801–23. - PubMed
-
- Hagen T, Korson MS, Wolfsdorf JI. Urinary lactate excretion to monitor the efficacy of treatment of type I glycogen storage disease. Mol Genet Metab. 2000;70:189–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

