Synaptosomal toxicity and nucleophilic targets of 4-hydroxy-2-nonenal
- PMID: 18996889
- PMCID: PMC2638640
- DOI: 10.1093/toxsci/kfn226
Synaptosomal toxicity and nucleophilic targets of 4-hydroxy-2-nonenal
Abstract
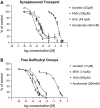
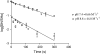
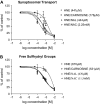
4-Hydroxy-2-nonenal (HNE) is an aldehyde by-product of lipid peroxidation that is presumed to play a primary role in certain neuropathogenic states (e.g., Alzheimer disease, spinal cord trauma). Although the molecular mechanism of neurotoxicity is unknown, proteomic analyses (e.g., tandem mass spectrometry) have demonstrated that this soft electrophile preferentially forms Michael-type adducts with cysteine sulfhydryl groups. In this study, we characterized HNE synaptosomal toxicity and evaluated the role of putative nucleophilic amino acid targets. Results show that HNE exposure of striatal synaptosomes inhibited (3)H-dopamine membrane transport and vesicular storage. These concentration-dependent effects corresponded to parallel decreases in synaptosomal sulfhydryl content. Calculations of quantum mechanical parameters (softness, electrophilicity) that describe the interactions of an electrophile with its nucleophilic target indicated that the relative softness of HNE was directly related to both the second-order rate constant (k(2)) for sulfhydryl adduct formation and corresponding neurotoxic potency (IC(50)). Computation of additional quantum mechanical parameters that reflect the relative propensity of a nucleophile to interact with a given electrophile (chemical potential, nucleophilicity) indicated that the sulfhydryl thiolate state was the HNE target. In support of this, we showed that the rate of adduct formation was related to pH and that N-acetyl-L-cysteine, but not N-acetyl-L-lysine or beta-alanyl-L-histidine, reduced in vitro HNE neurotoxicity. These data suggest that, like other type 2 alkenes, HNE produces nerve terminal toxicity by forming adducts with sulfhydryl thiolates on proteins involved in neurotransmission.
Figures
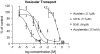

Similar articles
-
Reactions of electrophiles with nucleophilic thiolate sites: relevance to pathophysiological mechanisms and remediation.Free Radic Res. 2016;50(2):195-205. doi: 10.3109/10715762.2015.1094184. Epub 2015 Nov 11. Free Radic Res. 2016. PMID: 26559119 Free PMC article. Review.
-
Neurotoxic mechanisms of electrophilic type-2 alkenes: soft soft interactions described by quantum mechanical parameters.Toxicol Sci. 2007 Aug;98(2):561-70. doi: 10.1093/toxsci/kfm127. Epub 2007 May 22. Toxicol Sci. 2007. PMID: 17519395
-
Structure-toxicity analysis of type-2 alkenes: in vitro neurotoxicity.Toxicol Sci. 2007 Jan;95(1):136-46. doi: 10.1093/toxsci/kfl127. Epub 2006 Oct 5. Toxicol Sci. 2007. PMID: 17023561
-
Molecular mechanisms of 4-hydroxy-2-nonenal and acrolein toxicity: nucleophilic targets and adduct formation.Chem Res Toxicol. 2009 Sep;22(9):1499-508. doi: 10.1021/tx900147g. Chem Res Toxicol. 2009. PMID: 19610654 Free PMC article. Review.
-
Detoxification of cytotoxic alpha,beta-unsaturated aldehydes by carnosine: characterization of conjugated adducts by electrospray ionization tandem mass spectrometry and detection by liquid chromatography/mass spectrometry in rat skeletal muscle.J Mass Spectrom. 2002 Dec;37(12):1219-28. doi: 10.1002/jms.381. J Mass Spectrom. 2002. PMID: 12489081
Cited by
-
The 4-Hydroxynonenal-Protein Adducts and Their Biological Relevance: Are Some Proteins Preferred Targets?Antioxidants (Basel). 2023 Apr 1;12(4):856. doi: 10.3390/antiox12040856. Antioxidants (Basel). 2023. PMID: 37107229 Free PMC article. Review.
-
Reactions of electrophiles with nucleophilic thiolate sites: relevance to pathophysiological mechanisms and remediation.Free Radic Res. 2016;50(2):195-205. doi: 10.3109/10715762.2015.1094184. Epub 2015 Nov 11. Free Radic Res. 2016. PMID: 26559119 Free PMC article. Review.
-
Mass spectrometric evidence of malonaldehyde and 4-hydroxynonenal adductions to radical-scavenging soy peptides.J Agric Food Chem. 2012 Sep 26;60(38):9727-36. doi: 10.1021/jf3026277. Epub 2012 Sep 11. J Agric Food Chem. 2012. PMID: 22946674 Free PMC article.
-
β-dicarbonyl enolates: a new class of neuroprotectants.J Neurochem. 2011 Jan;116(1):132-43. doi: 10.1111/j.1471-4159.2010.07091.x. Epub 2010 Dec 2. J Neurochem. 2011. PMID: 21054388 Free PMC article.
-
Molecular mechanism of glyceraldehyde-3-phosphate dehydrogenase inactivation by α,β-unsaturated carbonyl derivatives.Chem Res Toxicol. 2011 Dec 19;24(12):2302-11. doi: 10.1021/tx200437y. Epub 2011 Nov 29. Chem Res Toxicol. 2011. PMID: 22084934 Free PMC article.
References
-
- Aldini G, Dalle-Donne I, Vistoli G, Facino RM, Carini M. Covalent modification of actin by 4-hydroxy-trans-2-nonenal (HNE): LC-ESI-MS/MS evidence for Cys374 Michael adduction. J. Mass Spectrom. 2005;40:946–954. - PubMed
-
- Barber DS, Stevens S, LoPachin RM. Proteomic analysis of rat striatal synaptosomes during acrylamide intoxication at a low dose rate. Toxicol. Sci. 2007;100:156–167. - PubMed
-
- Barber DS, LoPachin RM. Proteomic analysis of acrylamide-protein adduct formation in rat brain synaptosomes. Toxicol. Appl. Pharmacol. 2004;201:120–136. - PubMed
-
- Barford D. The role of cysteine residues as redox-sensitive regulatory switches. Curr. Opin. Struct. Biol. 2004;14:679–686. - PubMed
-
- Britto PJ, Knipling L, Wolff J. The local electrostatic environment determines cysteine reactivity of tubulin. J. Biol. Chem. 2002;277:29018–29027. - PubMed

