Disruption of neural progenitors along the ventricular and subventricular zones in periventricular heterotopia
- PMID: 18996916
- PMCID: PMC2722192
- DOI: 10.1093/hmg/ddn377
Disruption of neural progenitors along the ventricular and subventricular zones in periventricular heterotopia
Abstract
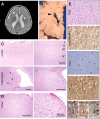
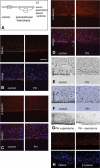
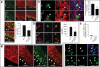
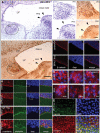
Periventricular heterotopia (PH) is a disorder characterized by neuronal nodules, ectopically positioned along the lateral ventricles of the cerebral cortex. Mutations in either of two human genes, Filamin A (FLNA) or ADP-ribosylation factor guanine exchange factor 2 (ARFGEF2), cause PH (Fox et al. in 'Mutations in filamin 1 prevent migration of cerebral cortical neurons in human periventricular heterotopia'. Neuron, 21, 1315-1325, 1998; Sheen et al. in 'Mutations in ARFGEF2 implicate vesicle trafficking in neural progenitor proliferation and migration in the human cerebral cortex'. Nat. Genet., 36, 69-76, 2004). Recent studies have shown that mutations in mitogen-activated protein kinase kinase kinase-4 (Mekk4), an indirect interactor with FlnA, also lead to periventricular nodule formation in mice (Sarkisian et al. in 'MEKK4 signaling regulates filamin expression and neuronal migration'. Neuron, 52, 789-801, 2006). Here we show that neurons in post-mortem human PH brains migrated appropriately into the cortex, that periventricular nodules were primarily composed of later-born neurons, and that the neuroependyma was disrupted in all PH cases. As studied in the mouse, loss of FlnA or Big2 function in neural precursors impaired neuronal migration from the germinal zone, disrupted cell adhesion and compromised neuroepithelial integrity. Finally, the hydrocephalus with hop gait (hyh) mouse, which harbors a mutation in Napa [encoding N-ethylmaleimide-sensitive factor attachment protein alpha (alpha-SNAP)], also develops a progressive denudation of the neuroepithelium, leading to periventricular nodule formation. Previous studies have shown that Arfgef2 and Napa direct vesicle trafficking and fusion, whereas FlnA associates dynamically with the Golgi membranes during budding and trafficking of transport vesicles. Our current findings suggest that PH formation arises from a final common pathway involving disruption of vesicle trafficking, leading to impaired cell adhesion and loss of neuroependymal integrity.
Figures




Similar articles
-
Overlapping expression of ARFGEF2 and Filamin A in the neuroependymal lining of the lateral ventricles: insights into the cause of periventricular heterotopia.J Comp Neurol. 2006 Jan 20;494(3):476-84. doi: 10.1002/cne.20806. J Comp Neurol. 2006. PMID: 16320251
-
A glial origin for periventricular nodular heterotopia caused by impaired expression of Filamin-A.Hum Mol Genet. 2012 Mar 1;21(5):1004-17. doi: 10.1093/hmg/ddr531. Epub 2011 Nov 10. Hum Mol Genet. 2012. PMID: 22076441
-
Filamin A regulates neuronal migration through brefeldin A-inhibited guanine exchange factor 2-dependent Arf1 activation.J Neurosci. 2013 Oct 2;33(40):15735-46. doi: 10.1523/JNEUROSCI.1939-13.2013. J Neurosci. 2013. PMID: 24089482 Free PMC article.
-
Trouble making the first move: interpreting arrested neuronal migration in the cerebral cortex.Trends Neurosci. 2008 Feb;31(2):54-61. doi: 10.1016/j.tins.2007.11.009. Epub 2008 Jan 16. Trends Neurosci. 2008. PMID: 18201775 Review.
-
Periventricular heterotopia.Epilepsy Behav. 2005 Sep;7(2):143-9. doi: 10.1016/j.yebeh.2005.05.001. Epilepsy Behav. 2005. PMID: 15996530 Review.
Cited by
-
Cytoskeletal proteins in cortical development and disease: actin associated proteins in periventricular heterotopia.Front Cell Neurosci. 2015 Apr 1;9:99. doi: 10.3389/fncel.2015.00099. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25883548 Free PMC article. Review.
-
Mutations in the HECT domain of NEDD4L lead to AKT-mTOR pathway deregulation and cause periventricular nodular heterotopia.Nat Genet. 2016 Nov;48(11):1349-1358. doi: 10.1038/ng.3676. Epub 2016 Oct 3. Nat Genet. 2016. PMID: 27694961 Free PMC article.
-
Movement disorder and neuronal migration disorder due to ARFGEF2 mutation.Neurogenetics. 2009 Oct;10(4):333-6. doi: 10.1007/s10048-009-0192-2. Epub 2009 Apr 22. Neurogenetics. 2009. PMID: 19384555 Free PMC article.
-
Neuroimaging in Pediatric Hydrocephalus.Indian J Pediatr. 2019 Oct;86(10):952-960. doi: 10.1007/s12098-019-02962-z. Epub 2019 May 10. Indian J Pediatr. 2019. PMID: 31077004 Review.
-
Filamin a regulates neural progenitor proliferation and cortical size through Wee1-dependent Cdk1 phosphorylation.J Neurosci. 2012 May 30;32(22):7672-84. doi: 10.1523/JNEUROSCI.0894-12.2012. J Neurosci. 2012. PMID: 22649246 Free PMC article.
References
-
- Barkovich A.J., Koch T.K., Carrol C.L. The spectrum of lissencephaly: report of ten patients analyzed by magnetic resonance imaging. Ann. Neurol. 1991;30:139–146. - PubMed
-
- Dobyns W.B., Reiner O., Carrozzo R., Ledbetter D.H. Lissencephaly. A human brain malformation associated with deletion of the LIS1 gene located at chromosome 17p13. JAMA. 1993;270:2838–2842. - PubMed
-
- Dobyns W.B., Andermann E., Andermann F., Czapansky-Beilman D., Dubeau F., Dulac O., Guerrini R., Hirsch B., Ledbetter D.H., Lee N.S., et al. X-linked malformations of neuronal migration. Neurology. 1996;47:331–339. - PubMed
-
- Hashimoto R., Seki T., Takuma Y., Suzuki N. The ‘double cortex’ syndrome on MRI. Brain Dev. 1993;15:57–59. discussion: 83–84. - PubMed
-
- Ricci S., Cusmai R., Fariello G., Fusco L., Vigevano F. Double cortex. A neuronal migration anomaly as a possible cause of Lennox–Gastaut syndrome. Arch. Neurol. 1992;49:61–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

