Defective domain-domain interactions within the ryanodine receptor as a critical cause of diastolic Ca2+ leak in failing hearts
- PMID: 18996969
- PMCID: PMC2721653
- DOI: 10.1093/cvr/cvn303
Defective domain-domain interactions within the ryanodine receptor as a critical cause of diastolic Ca2+ leak in failing hearts
Abstract
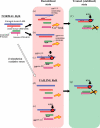
Aims: A domain peptide (DP) matching the Gly(2460)-Pro(2495) region of the cardiac type-2 ryanodine receptor (RyR2), DPc10, is known to mimic channel dysfunction associated with catecholaminergic polymorphic ventricular tachycardia (CPVT), owing to its interference in a normal interaction of the N-terminal (1-600) and central (2000-2500) domains (viz. domain unzipping). Using DPc10 and two other DPs harboring different mutation sites, we investigated the underlying mechanism of abnormal Ca(2+) cycling in failing hearts.
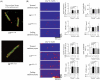
Methods and results: Sarcoplasmic reticulum (SR) vesicles and cardiomyocytes were isolated from dog left ventricular muscles for Ca(2+) leak and spark assays. The RyR2 moiety of the SR was fluorescently labelled with methylcoumarin acetate (MCA) using DPs corresponding to the 163-195 and 4090-4123 regions of RyR2 (DP163-195 and DP4090-4123, respectively) as site-directed carriers. Both DPs mediated a specific MCA fluorescence labelling of RyR2. Addition of either DP to the MCA-labelled SR induced domain unzipping, as evidenced by an increased accessibility of the bound MCA to a large-size fluorescence quencher. Both SR Ca(2+) leak and Ca(2+) spark frequency (SpF) were markedly increased in failing cardiomyocytes. Upon introduction of DP163-195 or DP4090-4123 into normal SR or cardiomyocytes, both Ca(2+) leak and SpF increased to the levels comparable with those of failing myocytes. K201 (JTV519) suppressed all of the effects induced by DP163-195 (domain unzipping and increased Ca(2+) leak and SpF) or those in failing cardiomyocytes, but did not suppress the effects induced by DP4090-4123.
Conclusion: Defective inter-domain interaction between N-terminal and central domains induces diastolic Ca(2+) leak, leading to heart failure and lethal arrhythmia. Mutation at the C-terminal region seen in CPVT does not seem to communicate with the aforementioned N-terminal and central inter-domain interaction, although spontaneous Ca(2+) leak is similarly induced.
Figures





Similar articles
-
Identification of target domains of the cardiac ryanodine receptor to correct channel disorder in failing hearts.Circulation. 2008 Feb 12;117(6):762-72. doi: 10.1161/CIRCULATIONAHA.107.718957. Epub 2008 Jan 28. Circulation. 2008. PMID: 18227387
-
Defective regulation of interdomain interactions within the ryanodine receptor plays a key role in the pathogenesis of heart failure.Circulation. 2005 Jun 28;111(25):3400-10. doi: 10.1161/CIRCULATIONAHA.104.507921. Epub 2005 Jun 20. Circulation. 2005. PMID: 15967847
-
Dissociation of calmodulin from cardiac ryanodine receptor causes aberrant Ca(2+) release in heart failure.Cardiovasc Res. 2010 Sep 1;87(4):609-17. doi: 10.1093/cvr/cvq108. Epub 2010 Apr 13. Cardiovasc Res. 2010. PMID: 20388639 Free PMC article.
-
Sarcoplasmic reticulum calcium leak and cardiac arrhythmias.Biochem Soc Trans. 2007 Nov;35(Pt 5):952-6. doi: 10.1042/BST0350952. Biochem Soc Trans. 2007. PMID: 17956253 Review.
-
Intracellular calcium leak in heart failure and atrial fibrillation: a unifying mechanism and therapeutic target.Nat Rev Cardiol. 2020 Nov;17(11):732-747. doi: 10.1038/s41569-020-0394-8. Epub 2020 Jun 18. Nat Rev Cardiol. 2020. PMID: 32555383 Free PMC article. Review.
Cited by
-
Enhancing calmodulin binding to cardiac ryanodine receptor completely inhibits pressure-overload induced hypertrophic signaling.Commun Biol. 2020 Nov 26;3(1):714. doi: 10.1038/s42003-020-01443-w. Commun Biol. 2020. PMID: 33244105 Free PMC article.
-
Cardiac ventricular repolarization reserve: a principle for understanding drug-related proarrhythmic risk.Br J Pharmacol. 2011 Sep;164(1):14-36. doi: 10.1111/j.1476-5381.2011.01367.x. Br J Pharmacol. 2011. PMID: 21545574 Free PMC article. Review.
-
Dantrolene rescues arrhythmogenic RYR2 defect in a patient-specific stem cell model of catecholaminergic polymorphic ventricular tachycardia.EMBO Mol Med. 2012 Mar;4(3):180-91. doi: 10.1002/emmm.201100194. Epub 2012 Jan 25. EMBO Mol Med. 2012. PMID: 22174035 Free PMC article.
-
Novel mutations in arrhythmogenic right ventricular cardiomyopathy from Indian population.Indian J Hum Genet. 2011 May;17(2):70-6. doi: 10.4103/0971-6866.86182. Indian J Hum Genet. 2011. PMID: 22090716 Free PMC article.
-
IP(3) receptors: toward understanding their activation.Cold Spring Harb Perspect Biol. 2010 Dec;2(12):a004010. doi: 10.1101/cshperspect.a004010. Epub 2010 Oct 27. Cold Spring Harb Perspect Biol. 2010. PMID: 20980441 Free PMC article. Review.
References
-
- Yano M, Yamamoto T, Ikemoto N, Matsuzaki M. Abnormal Ryanodine Receptor Function in Heart Failure. (Review) Pharmacol Therapeut. 2005;107:377–391. - PubMed
-
- Wehrens XH, Lehnart SE, Huang F, Vest JA, Reiken SR, Mohler PJ, et al. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003;113:829–840. - PubMed
-
- Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): Defective regulation in failing hearts. Cell. 2000;101:365–376. - PubMed
-
- Jiang MT, Lokuta AJ, Farrell EF, Wolff MR, Haworth RA, Valdivia HH. Abnormal Ca2+ release, but normal ryanodine receptors, in canine and human heart failure. Circ Res. 2002;91:1015–1022. - PubMed
-
- Xiao B, Sutherland C, Walsh MP, Chen SR. Protein kinase A phosphorylation at serine-2808 of the cardiac Ca2+-release channel (ryanodine receptor) does not dissociate 12.6-kDa FK506-binding protein (FKBP12.6) Circ Res. 2004;94:487–495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

