Bacteria possessing two RelA/SpoT-like proteins have evolved a specific stringent response involving the acyl carrier protein-SpoT interaction
- PMID: 18996989
- PMCID: PMC2620808
- DOI: 10.1128/JB.01195-08
Bacteria possessing two RelA/SpoT-like proteins have evolved a specific stringent response involving the acyl carrier protein-SpoT interaction
Abstract
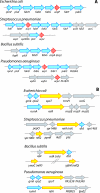
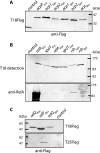
Bacteria respond to nutritional stress by producing (p)ppGpp, which triggers a stringent response resulting in growth arrest and expression of resistance genes. In Escherichia coli, RelA produces (p)ppGpp upon amino acid starvation by detecting stalled ribosomes. The SpoT enzyme responds to various other types of starvation by unknown mechanisms. We previously described an interaction between SpoT and the central cofactor of lipid synthesis, acyl carrier protein (ACP), which is involved in detecting starvation signals in lipid metabolism and triggering SpoT-dependent (p)ppGpp accumulation. However, most bacteria possess a unique protein homologous to RelA/SpoT (Rsh) that is able to synthesize and degrade (p)ppGpp and is therefore more closely related to SpoT function. In this study, we asked if the ACP-SpoT interaction is specific for bacteria containing two RelA and SpoT enzymes or if it is a general feature that is conserved in Rsh enzymes. By testing various combinations of SpoT, RelA, and Rsh enzymes and ACPs of E. coli, Pseudomonas aeruginosa, Bacillus subtilis and Streptococcus pneumoniae, we found that the interaction between (p)ppGpp synthases and ACP seemed to be restricted to SpoT proteins of bacteria containing the two RelA and SpoT proteins and to ACP proteins encoded by genes located in fatty acid synthesis operons. When Rsh enzymes from B. subtilis and S. pneumoniae are produced in E. coli, the behavior of these enzymes is different from the behavior of both RelA and SpoT proteins with respect to (p)ppGpp synthesis. This suggests that bacteria have evolved several different modes of (p)ppGpp regulation in order to respond to nutrient starvation.
Figures


References
-
- Battesti, A., and E. Bouveret. 2006. Acyl carrier protein/SpoT interaction, the switch linking SpoT-dependent stress response to fatty acid metabolism. Mol. Microbiol. 621048-1063. - PubMed
-
- Battesti, A., and E. Bouveret. 2008. Improvement of bacterial two-hybrid vectors for detection of fusion proteins and transfer to PBAD-tandem affinity purification, calmodulin binding peptide, or 6-histidine tag vectors. Proteomics 84768-4771. - PubMed
-
- Cashel, M. 1994. Detection of (p)ppGpp accumulation patterns in Escherichia coli mutants. Methods Mol. Genet. 3341-356.
-
- Cashel, M., D. Gentry, V. J. Hernandez, and D. Vinella. 1996. The stringent response, p. 1458-1496. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, DC.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

