Small-molecule type III secretion system inhibitors block assembly of the Shigella type III secreton
- PMID: 18996990
- PMCID: PMC2620818
- DOI: 10.1128/JB.01004-08
Small-molecule type III secretion system inhibitors block assembly of the Shigella type III secreton
Abstract
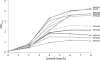
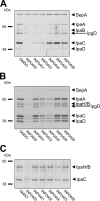
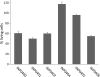
Type III secretion systems (T3SSs) are essential virulence devices for many gram-negative bacteria that are pathogenic for plants, animals, and humans. They serve to translocate virulence effector proteins directly into eukaryotic host cells. T3SSs are composed of a large cytoplasmic bulb and a transmembrane region into which a needle is embedded, protruding above the bacterial surface. The emerging antibiotic resistance of bacterial pathogens urges the development of novel strategies to fight bacterial infections. Therapeutics that rather than kill bacteria only attenuate their virulence may reduce the frequency or progress of resistance emergence. Recently, a group of salicylidene acylhydrazides were identified as inhibitors of T3SSs in Yersinia, Chlamydia, and Salmonella species. Here we show that these are also effective on the T3SS of Shigella flexneri, where they block all related forms of protein secretion so far known, as well as the epithelial cell invasion and induction of macrophage apoptosis usually demonstrated by this bacterium. Furthermore, we show the first evidence for the detrimental effect of these compounds on T3SS needle assembly, as demonstrated by increased numbers of T3S apparatuses without needles or with shorter needles. Therefore, the compounds generate a phenocopy of T3SS export apparatus mutants but with incomplete penetrance. We discuss why this would be sufficient to almost completely block the later secretion of effector proteins and how this begins to narrow the search for the molecular target of these compounds.
Figures


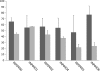
References
-
- Bailey, L., A. Gylfe, C. Sundin, S. Muschiol, M. Elofsson, P. Nordstrom, B. Henriques-Normark, R. Lugert, A. Waldenstrom, H. Wolf-Watz, and S. Bergstrom. 2007. Small molecule inhibitors of type III secretion in Yersinia block the Chlamydia pneumoniae infection cycle. FEBS Lett. 581587-595. - PubMed
-
- Blocker, A., N. Jouihri, E. Larquet, P. Gounon, F. Ebel, C. Parsot, P. Sansonetti, and A. Allaoui. 2001. Structure and composition of the Shigella flexneri “needle complex,” a part of its type III secreton. Mol. Microbiol. 39652-663. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

