Two protonation switches control rhodopsin activation in membranes
- PMID: 18997017
- PMCID: PMC2584695
- DOI: 10.1073/pnas.0804541105
Two protonation switches control rhodopsin activation in membranes
Abstract
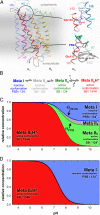
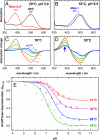
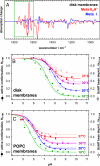
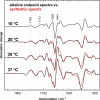
Activation of the G protein-coupled receptor (GPCR) rhodopsin is initiated by light-induced isomerization of the retinal ligand, which triggers 2 protonation switches in the conformational transition to the active receptor state Meta II. The first switch involves disruption of an interhelical salt bridge by internal proton transfer from the retinal protonated Schiff base (PSB) to its counterion, Glu-113, in the transmembrane domain. The second switch consists of uptake of a proton from the solvent by Glu-134 of the conserved E(D)RY motif at the cytoplasmic terminus of helix 3, leading to pH-dependent receptor activation. By using a combination of UV-visible and FTIR spectroscopy, we study the activation mechanism of rhodopsin in different membrane environments and show that these 2 protonation switches become partially uncoupled at physiological temperature. This partial uncoupling leads to approximately 50% population of an entropy-stabilized Meta II state in which the interhelical PSB salt bridge is broken and activating helix movements have taken place but in which Glu-134 remains unprotonated. This partial activation is converted to full activation only by coupling to the pH-dependent protonation of Glu-134 from the solvent, which stabilizes the active receptor conformation by lowering its enthalpy. In a membrane environment, protonation of Glu-134 is therefore a thermodynamic rather than a structural prerequisite for activating helix movements. In light of the conservation of the E(D)RY motif in rhodopsin-like GPCRs, protonation of this carboxylate also may serve a similar function in signal transduction of other members of this receptor family.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Samama P, Cotecchia S, Costa T, Lefkowitz RJ. A mutation-induced activated state of the beta 2-adrenergic receptor. Extending the ternary complex model. J Biol Chem. 1993;268:4625–4636. - PubMed
-
- Menon ST, Han M, Sakmar TP. Rhodopsin: Structural basis of molecular physiology. Physiol Rev. 2001;81:1659–1688. - PubMed
-
- Lewis JW, Kliger DS. Absorption spectroscopy in studies of visual pigments: Spectral and kinetic characterization of intermediates. Methods Enzymol. 2000;315:164–178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

