Time and dose dependence of pluronic bioactivity in hyperthermia-induced tumor cell death
- PMID: 18997100
- PMCID: PMC3293626
- DOI: 10.3181/0807-RM-223
Time and dose dependence of pluronic bioactivity in hyperthermia-induced tumor cell death
Abstract
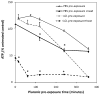
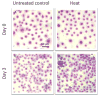
Pluronic block copolymers have been shown to sensitize cancer cells resulting in an increased activity of antineoplastic agents. In the current study we examined a new application of Pluronic bioactivity in potentiating hyperthermia-induced cancer cell injury. DHD/K12/TRb rat adenocarcinoma cells were exposed to low-grade hyperthermia at 43 degrees C with or without Pluronic P85 or Pluronic L61. A range of Pluronic doses, pre-exposure and heat exposure durations were investigated, and the test conditions were optimized. Treatment efficacy was assessed by measurement of intracellular ATP and mitochondrial dehydrogenase activity. Both P85 and L61 in synergy with heat reduced cell viability appreciably compared to either heat or Pluronic alone. Under optimal conditions, P85 (10 mg/ml, 240 mins) combined with 15 mins heat reduced intracellular ATP to 60.1 +/- 3.5% of control, while heat alone and P85 without heat caused a negligible decrease in ATP of 1.2% and 3.8%, respectively. Similarly, cells receiving 120 mins pre-exposure of L61 (0.3 mg/ml) showed reduction in intracellular ATP to 14.1 +/- 2.1% of control. Again, heat or L61 pre-exposure alone caused a minor decrease in levels of intracellular ATP (1.5% and 4.4%, respectively). Comparable results were observed when viability was assessed by mitochondrial enzyme activity. Survival studies confirmed that the loss of viability translates to a long-term reduction in proliferative activity, particularly for L61 treated cells. Based on these results, we conclude that Pluronic is effective in improving hyperthermic cancer treatment in vitro by potentiating heat-induced cytotoxicity in a concentration and time dependent manner.
Figures




Similar articles
-
Role of Pluronic block copolymers in modulation of heat shock protein 70 expression.Int J Hyperthermia. 2011;27(7):672-81. doi: 10.3109/02656736.2011.608218. Int J Hyperthermia. 2011. PMID: 21992560 Free PMC article.
-
Effect of intratumoral injection of carboplatin combined with pluronic P85 or L61 on experimental colorectal carcinoma in rats.Exp Biol Med (Maywood). 2007 Jul;232(7):950-7. Exp Biol Med (Maywood). 2007. PMID: 17609512
-
Enhancement of carboplatin toxicity by Pluronic block copolymers.J Control Release. 2005 Aug 18;106(1-2):188-97. doi: 10.1016/j.jconrel.2005.04.015. J Control Release. 2005. PMID: 15951044
-
An essential relationship between ATP depletion and chemosensitizing activity of Pluronic block copolymers.J Control Release. 2003 Aug 28;91(1-2):75-83. doi: 10.1016/s0168-3659(03)00211-6. J Control Release. 2003. PMID: 12932639 Free PMC article. Review.
-
Pluronic block copolymers for overcoming drug resistance in cancer.Adv Drug Deliv Rev. 2002 Sep 13;54(5):759-79. doi: 10.1016/s0169-409x(02)00047-9. Adv Drug Deliv Rev. 2002. PMID: 12204601 Review.
Cited by
-
Phase-shift, stimuli-responsive perfluorocarbon nanodroplets for drug delivery to cancer.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012 Sep-Oct;4(5):492-510. doi: 10.1002/wnan.1176. Epub 2012 Jun 22. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012. PMID: 22730185 Free PMC article. Review.
-
Formulation and characterization of echogenic lipid-Pluronic nanobubbles.Mol Pharm. 2010 Feb 1;7(1):49-59. doi: 10.1021/mp9001816. Mol Pharm. 2010. PMID: 19957968 Free PMC article.
-
Role of Pluronic block copolymers in modulation of heat shock protein 70 expression.Int J Hyperthermia. 2011;27(7):672-81. doi: 10.3109/02656736.2011.608218. Int J Hyperthermia. 2011. PMID: 21992560 Free PMC article.
-
Phase-shift, stimuli-responsive drug carriers for targeted delivery.Ther Deliv. 2011 Sep;2(9):1165-87. doi: 10.4155/tde.11.81. Ther Deliv. 2011. PMID: 22059114 Free PMC article. Review.
-
Structural parameters governing activity of Pluronic triblock copolymers in hyperthermia cancer therapy.Int J Hyperthermia. 2011;27(7):663-71. doi: 10.3109/02656736.2011.599828. Int J Hyperthermia. 2011. PMID: 21992559 Free PMC article.
References
-
- Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, Felix R, Riess H. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol. 2002;43:33–56. - PubMed
-
- Chen B, Roskams T, de Witte PA. Enhancing the antitumoral effect of hypericin-mediated photodynamic therapy by hyperthermia. Lasers Surg Med. 2002;31:158–163. - PubMed
-
- Mayhew JF. Intraoperative hyperthermia in a child with neuroblastoma. Paediatr Anaesth. 2006;16:890–891. - PubMed
-
- Schlemmer M, Lindner LH, Abdel-Rahman S, Issels RD. Principles, technology and indication of hyperthermia and part body hyperthermia. Radiologe. 2004;44:301–309. - PubMed
-
- Mambrini A, Del Freo A, Pacetti P, Orlandi M, Torri T, Fiorentini G, Cantore M. Intra-arterial and systemic chemotherapy plus external hyperthermia in unresectable biliary cancer. Clin Oncol (R Coll Radiol) 2007;19:805–806. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

