Divergent regulation of terpenoid metabolism in the trichomes of wild and cultivated tomato species
- PMID: 18997116
- PMCID: PMC2613740
- DOI: 10.1104/pp.108.126276
Divergent regulation of terpenoid metabolism in the trichomes of wild and cultivated tomato species
Abstract
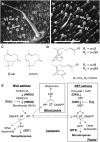
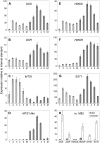
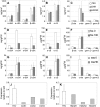
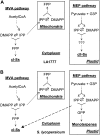
The diversification of chemical production in glandular trichomes is important in the development of resistance against pathogens and pests in two species of tomato. We have used genetic and genomic approaches to uncover some of the biochemical and molecular mechanisms that underlie the divergence in trichome metabolism between the wild species Solanum habrochaites LA1777 and its cultivated relative, Solanum lycopersicum. LA1777 produces high amounts of insecticidal sesquiterpene carboxylic acids (SCAs), whereas cultivated tomatoes lack SCAs and are more susceptible to pests. We show that trichomes of the two species have nearly opposite terpenoid profiles, consisting mainly of monoterpenes and low levels of sesquiterpenes in S. lycopersicum and mainly of SCAs and very low monoterpene levels in LA1777. The accumulation patterns of these terpenoids are different during development, in contrast to the developmental expression profiles of terpenoid pathway genes, which are similar in the two species, but they do not correlate in either case with terpenoid accumulation. However, our data suggest that the accumulation of monoterpenes in S. lycopersicum and major sesquiterpenes in LA1777 are linked both genetically and biochemically. Metabolite analyses after targeted gene silencing, inhibitor treatments, and precursor feeding all show that sesquiterpene biosynthesis relies mainly on products from the plastidic 2-C-methyl-d-erythritol-4-phosphate pathway in LA1777 but less so in the cultivated species. Furthermore, two classes of sesquiterpenes produced by the wild species may be synthesized from distinct pools of precursors via cytosolic and plastidial cyclases. However, highly trichome-expressed sesquiterpene cyclase-like enzymes were ruled out as being involved in the production of major LA1777 sesquiterpenes.
Figures


References
-
- Adam KP, Thiel R, Zapp J (1999) Incorporation of 1-[1-(13)C]deoxy-D-xylulose in chamomile sesquiterpenes. Arch Biochem Biophys 369 127–132 - PubMed
-
- Adams RP (2004) Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy. Allured Publishing, Carol Stream, IL
-
- Akhila A, Sharma PK, Thakur RS (1986) A novel biosynthesis of irregular sesquiterpene artemone in Artemisia pallens. Tetrahedron Lett 27 5885–5888
-
- Aubourg S, Lecharny A, Bohlmann J (2002) Genomic analysis of the terpenoid synthase (AtTPS) gene family of Arabidopsis thaliana. Mol Genet Genomics 267 730–745 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

