Continuous shifts in the active set of spinal interneurons during changes in locomotor speed
- PMID: 18997790
- PMCID: PMC2735137
- DOI: 10.1038/nn.2225
Continuous shifts in the active set of spinal interneurons during changes in locomotor speed
Abstract
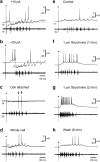
The classic 'size principle' of motor control describes how increasingly forceful movements arise by the recruitment of motoneurons of progressively larger size and force output into the active pool. We explored the activity of pools of spinal interneurons in larval zebrafish and found that increases in swimming speed were not associated with the simple addition of cells to the active pool. Instead, the recruitment of interneurons at faster speeds was accompanied by the silencing of those driving movements at slower speeds. This silencing occurred both between and within classes of rhythmically active premotor excitatory interneurons. Thus, unlike motoneurons, there is a continuous shift in the set of cells driving the behavior, even though changes in the speed of the movements and the frequency of the motor pattern appear to be smoothly graded. We conclude that fundamentally different principles may underlie the recruitment of motoneuron and interneuron pools.
Figures








Comment in
-
Switching gears in the spinal cord.Nat Neurosci. 2008 Dec;11(12):1367-8. doi: 10.1038/nn1208-1367. Nat Neurosci. 2008. PMID: 19023340 No abstract available.
References
-
- Mendell LM. The size principle: a rule describing the recruitment of motoneurons. J Neurophysiol. 2005;93:3024–3026. - PubMed
-
- Cope TC, Sokoloff AJ. Orderly recruitment among motoneurons supplying different muscles. J Physiol Paris. 1999;93:81–85. - PubMed
-
- Cope TC, Pinter MJ. The size principle: Still working after all these years. News in Physiological Sciences. 1995;10:280–286.
-
- Henneman E, Somjen G, Carpenter DO. Functional Significance of Cell Size in Spinal Motoneurons. J Neurophysiol. 1965;28:560–580. - PubMed
-
- Zajac FE, Faden JS. Relationship among recruitment order, axonal conduction velocity, and muscle-unit properties of type-identified motor units in cat plantaris muscle. J Neurophysiol. 1985;53:1303–1322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

