Synthesis and structure revision of nakiterpiosin
- PMID: 18998640
- PMCID: PMC2635422
- DOI: 10.1021/ja808110d
Synthesis and structure revision of nakiterpiosin
Abstract
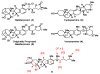
This manuscript describes a convergent synthesis and the revision of the relative stereochemistry of nakiterpiosin, a marine C-nor-D-homosteroid. Our synthesis features a late-stage carbonylative Stille cross-coupling reaction and a photo-Nazarov cyclization reaction that deliver the complete nakiterpiosin skeleton efficiently.
Figures

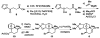
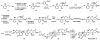
References
-
- Teruya T, Nakagawa S, Koyama T, Arimoto H, Kita M, Uemura D. Tetrahedron. 2004;60:6989–6993.
- Teruya T, Nakagawa S, Koyama T, Suenaga K, Kita M, Uemura D. Tetrahedron Lett. 2003;44:5171–5173.
-
-
For a report of the synthetic studies toward nakiterpiosin, see: Ito T, Ito M, Arimoto H, Takamura H, Uemura D. Tetrahedron. 2007;48:5465–5469.
-
-
-
For a review on structure revisions of natural products through synthetic studies, see: Nicolaou KC, Snyder SA. Angew Chem Int Ed. 2005;44:1012–1044.
-
-
-
Cyclopamine is also known as 11-deoxojervine. For the seminal semi-synthesis of jervine and veratramine, see: Masamune T, Takasugi M, Murai A, Kobayashi K. J Am Chem Soc. 1967;89:4521–4523.Johnson WS, deJongh HAP, Coverdale CE, Scott JW, Burckhardt U. J Am Chem Soc. 1967;89:4523–4524.Masamune T, Takasugi M, Murai A. Tetrahedron. 1971;27:3369–3386.Kutney JP, Cable J, Gladstone WAF, Hanssen HW, Nair GV, Torupka EJ, Warnock WDC. Can J Chem. 1976;53:1796–1817.
-
-
-
For reviews, see: McFerren MA. J Am Acad Dermatol. 2006;54:718–720.Keeler RF. Lipids. 1978;13:708–715.James LF. J Nat Toxins. 1999;8:63–80.Binns W, James LF, Keeler RF, Balls LD. Cancer Res. 1968;28:2323–2326.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

