Compartmentalization of redox signaling through NADPH oxidase-derived ROS
- PMID: 18999986
- PMCID: PMC2842113
- DOI: 10.1089/ars.2008.2333
Compartmentalization of redox signaling through NADPH oxidase-derived ROS
Abstract
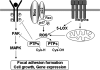
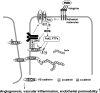
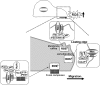
Reactive oxygen species (ROS) are generated in response to growth factors, cytokines, G protein-coupled receptor agonists, or shear stress, and function as signaling molecules in nonphagocytes. However, it is poorly understood how freely diffusible ROS can activate specific signaling, so-called "redox signaling." NADPH oxidases are a major source of ROS and now recognized to have specific subcellular localizations, and this targeting to specific compartments is required for localized ROS production. One important mechanism may involve the interaction of oxidase subunits with various targeting proteins localized in lamellipodial leading edge and focal adhesions/complexes. ROS are believed to inactivate protein tyrosine phosphatases, thereby establishing a positive-feedback system that promotes activation of specific redox signaling pathways involved in various functions. Additionally, ROS production may be localized through interactions of NADPH oxidase with signaling platforms associated with caveolae/lipid rafts, endosomes, and nucleus. These indicate that the specificity of ROS-mediated signal transduction may be modulated by the localization of Nox isoforms and their regulatory subunits within specific subcellular compartments. This review summarizes the recent progress on compartmentalization of redox signaling via activation of NADPH oxidase, which is implicated in cell biology and pathophysiologies.
Figures
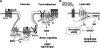
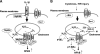
References
-
- Abate C. Patel L. Rauscher FJ., 3rd Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science (New York) 1990;249:1157–1161. - PubMed
-
- Abid MR. Kachra Z. Spokes KC. Aird WC. NADPH oxidase activity is required for endothelial cell proliferation and migration. FEBS Lett. 2000;486:252–256. - PubMed
-
- Allen RG. Tresini M. Oxidative stress and gene regulation. Free Radic Biol Med. 2000;28:463–499. - PubMed
-
- Allingham MJ. van Buul JD. Burridge K. ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J Immunol. 2007;179:4053–4064. - PubMed
-
- Ambasta RK. Kumar P. Griendling KK. Schmidt HH. Busse R. Brandes RP. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem. 2004;279:45935–45941. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

