Metabolism of 1alpha-hydroxyvitamin D3 by cytochrome P450scc to biologically active 1alpha,20-dihydroxyvitamin D3
- PMID: 19000766
- PMCID: PMC2605774
- DOI: 10.1016/j.jsbmb.2008.10.005
Metabolism of 1alpha-hydroxyvitamin D3 by cytochrome P450scc to biologically active 1alpha,20-dihydroxyvitamin D3
Abstract
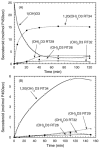
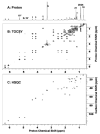
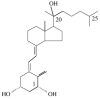
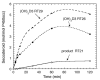
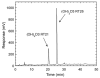
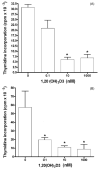
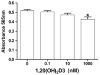
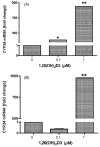
Cytochrome P450scc (CYP11A1) metabolizes vitamin D3 to 20-hydroxyvitamin D3 as the major product, with subsequent production of dihydroxy and trihydroxy derivatives. The aim of this study was to determine whether cytochrome P450scc could metabolize 1alpha-hydroxyvitamin D3 and whether products were biologically active. The major product of 1alpha-hydroxyvitamin D3 metabolism by P450scc was identified by mass spectrometry and NMR as 1alpha,20-dihydroxyvitamin D3. Mass spectrometry of minor metabolites revealed the production of another dihydroxyvitamin D3 derivative, two trihydroxy-metabolites made via 1alpha,20-dihydroxyvitamin D3 and a tetrahydroxyvitamin D3 derivative. The Km for 1alpha-hydroxyvitamin D3 determined for P450scc incorporated into phospholipid vesicles was 1.4 mol substrate/mol phospholipid, half that observed for vitamin D3. The kcat was 3.0 mol/min/mol P450scc, 6-fold lower than that for vitamin D3. 1alpha,20-Dihydroxyvitamin D3 inhibited DNA synthesis by human epidermal HaCaT keratinocytes propagated in culture, in a time- and dose-dependent fashion, with a potency similar to that of 1alpha,25-dihydroxyvitamin D3. 1alpha,20-Dihydroxyvitamin D3 (10 microM) enhanced CYP24 mRNA levels in HaCaT keratinocytes but the potency was much lower than that reported for 1alpha,25-dihydroxyvitamin D3. We conclude that the presence of the 1-hydroxyl group in vitamin D3 does not alter the major site of hydroxylation by P450scc which, as for vitamin D3, is at C20. The major product, 1alpha,20-dihydroxyvitamin D3, displays biological activity on keratinocytes and therefore might be useful pharmacologically.
Figures

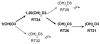
Similar articles
-
Production of 22-hydroxy metabolites of vitamin d3 by cytochrome p450scc (CYP11A1) and analysis of their biological activities on skin cells.Drug Metab Dispos. 2011 Sep;39(9):1577-88. doi: 10.1124/dmd.111.040071. Epub 2011 Jun 15. Drug Metab Dispos. 2011. PMID: 21677063 Free PMC article.
-
Pathways and products for the metabolism of vitamin D3 by cytochrome P450scc.FEBS J. 2008 May;275(10):2585-96. doi: 10.1111/j.1742-4658.2008.06406.x. Epub 2008 Apr 10. FEBS J. 2008. PMID: 18410379 Free PMC article.
-
An alternative pathway of vitamin D metabolism. Cytochrome P450scc (CYP11A1)-mediated conversion to 20-hydroxyvitamin D2 and 17,20-dihydroxyvitamin D2.FEBS J. 2006 Jul;273(13):2891-901. doi: 10.1111/j.1742-4658.2006.05302.x. FEBS J. 2006. PMID: 16817851 Free PMC article.
-
Enzymatic studies on the key enzymes of vitamin D metabolism; 1 alpha-hydroxylase (CYP27B1) and 24-hydroxylase (CYP24).Biotechnol Annu Rev. 2001;7:179-94. doi: 10.1016/s1387-2656(01)07037-5. Biotechnol Annu Rev. 2001. PMID: 11686044 Review.
-
The serum vitamin D metabolome: What we know and what is still to discover.J Steroid Biochem Mol Biol. 2019 Feb;186:4-21. doi: 10.1016/j.jsbmb.2018.09.003. Epub 2018 Sep 8. J Steroid Biochem Mol Biol. 2019. PMID: 30205156 Free PMC article. Review.
Cited by
-
Pilot study on the bioactivity of vitamin d in the skin after oral supplementation.Cancer Prev Res (Phila). 2015 Jun;8(6):563-9. doi: 10.1158/1940-6207.CAPR-14-0280. Epub 2015 Apr 2. Cancer Prev Res (Phila). 2015. PMID: 25835512 Free PMC article.
-
Antifibrogenic Activities of CYP11A1-derived Vitamin D3-hydroxyderivatives Are Dependent on RORγ.Endocrinology. 2021 Jan 1;162(1):bqaa198. doi: 10.1210/endocr/bqaa198. Endocrinology. 2021. PMID: 33107570 Free PMC article.
-
Novel activities of CYP11A1 and their potential physiological significance.J Steroid Biochem Mol Biol. 2015 Jul;151:25-37. doi: 10.1016/j.jsbmb.2014.11.010. Epub 2014 Nov 13. J Steroid Biochem Mol Biol. 2015. PMID: 25448732 Free PMC article. Review.
-
Detection of novel CYP11A1-derived secosteroids in the human epidermis and serum and pig adrenal gland.Sci Rep. 2015 Oct 8;5:14875. doi: 10.1038/srep14875. Sci Rep. 2015. PMID: 26445902 Free PMC article.
-
The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions.J Steroid Biochem Mol Biol. 2014 Oct;144 Pt A:28-39. doi: 10.1016/j.jsbmb.2013.10.012. Epub 2013 Oct 28. J Steroid Biochem Mol Biol. 2014. PMID: 24176765 Free PMC article. Review.
References
-
- Seino Y, Tanaka H, Yamaoka K, Yabuuchi H. Circulating 1 alpha,25-dihydroxyvitamin D levels after a single dose of 1 alpha,25-dihydroxyvitamin D3 or 1 alpha-hydroxyvitamin D3 in normal men. Bone Miner. 1987;2:479–485. - PubMed
-
- Aiba I, Yamasaki T, Shinki T, Izumi S, Yamamoto K, Yamada S, Terato H, Ide H, Ohyama Y. Characterization of rat and human CYP2J enzymes as Vitamin D 25-hydroxylases. Steroids. 2006;71:849–856. - PubMed
-
- Gupta RP, Hollis BW, Patel SB, Patrick KS, Bell NH. CYP3A4 is a human microsomal vitamin D 25-hydroxylase. J Bone Miner Res. 2004;19:680–688. - PubMed
-
- Shiraishi A, Higashi S, Ohkawa H, Kubodera N, Hirasawa T, Ezawa I, Ikeda K, Ogata E. The advantage of alfacalciferol over vitamin D in the treatment of osteoporosis. Calcif Tissue Int. 1999;65:311–316. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

