Coagulation dysregulation as a barrier to xenotransplantation in the primate
- PMID: 19000927
- PMCID: PMC2757551
- DOI: 10.1016/j.trim.2008.10.008
Coagulation dysregulation as a barrier to xenotransplantation in the primate
Abstract
Purpose of review: The ability to generate pigs expressing a human complement regulatory protein (hCRP) and/or pigs in which the alpha1,3-galactosyltransferase gene has been knocked out (GT-KO) has largely overcome the barrier of hyperacute rejection of a pig organ transplanted into a primate. However, acute humoral xenograft rejection (AHXR), presenting as microvascular thrombosis and/or consumptive coagulopathy, remains a major hurdle to successful xenotransplantation. This review summarizes recent studies of the coagulation problems associated with xenotransplantation, and discusses potential strategies to overcome them.
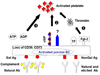
Recent progress: Organ transplantation into nonhuman primates from GT-KO pigs that express a hCRP are not susceptible to hyperacute rejection. Nevertheless, most recipients of GT-KO and/or hCRP transgenic pig organs develop a consumptive coagulopathy, even when the graft remains functioning. This is associated with platelet aggregation, thrombocytopenia, anemia, and a tendency to bleed. Whilst this may reflect an ongoing immune response against the graft, (as exposure to anti-nonGal antibodies in vitro induces procoagulant changes in porcine ECs, even in the absence of complement), histological examination of the graft often shows only minimal features of immune injury, unlike grafts undergoing typical AHXR. Importantly, recent in vitro studies have indicated that the coincubation of porcine endothelial cells (ECs) with human platelets activates the platelets to express tissue factor, independent of a humoral immune response. These observations suggest that the use of organs from GT-KO pigs that express a hCRP may not be sufficient to prevent the development of a coagulation disorder following xenotransplantation, even if complete immunological tolerance can be achieved.
Summary: Both thrombotic microangiopathy and systemic consumptive coagulopathy are increasingly recognized as barriers to successful xenotransplantation. The breeding of transgenic pigs with one or more human anticoagulant genes, such as CD39 or tissue factor pathway inhibitor, is anticipated to inhibit the procoagulant changes that take place on the graft ECs, and thus may prevent or reduce platelet activation that arises as a result of immune-mediated injury. The identification of the molecular mechanisms that develop between porcine ECs and human platelets may allow pharmacological approaches to be determined that inhibit the development of thrombotic microangiopathy and consumptive coagulopathy. Hopefully, further genetic modification of the organ-source pigs, combined with systemic drug therapy to the recipient, will prolong graft survival further.
Figures
References
-
- Cooper DK, Gollackner B, Sachs DH. Will the pig solve the transplantation backlog? Annu Rev Med. 2002;53:133–147. - PubMed
-
- Cooper DK, Dorling A, Pierson RN, 3rd, Rees M, Seebach J, Yazer M, Ohdan H, Awwad M, Ayares D. Alpha1,3-galactosyltransferase gene-knockout pigs for xenotransplantation: where do we go from here? Transplantation. 2007;84:1–7. - PubMed
-
- Mackman N. Tissue-specific hemostasis: role of tissue factor. J Thromb Haemost. 2008;6:303–305. - PubMed
-
- Schulte Am Esch J, 2nd, Robson SC, Knoefel WT, Hosch SB, Rogiers X. O-linked glycosylation and functional incompatibility of porcine von Willebrand factor for human platelet GPIb receptors. Xenotransplantation. 2005;12:30–37. - PubMed
-
- Kopp CW, Grey ST, Siegel JB, McShea A, Vetr H, Wrighton CJ, Schulte am Esch J, 2nd, Bach FH, Robson SC. Expression of human thrombomodulin cofactor activity in porcine endothelial cells. Transplantation. 1998;66:244–251. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

