Cx36 makes channels coupling human pancreatic beta-cells, and correlates with insulin expression
- PMID: 19000992
- PMCID: PMC2638800
- DOI: 10.1093/hmg/ddn370
Cx36 makes channels coupling human pancreatic beta-cells, and correlates with insulin expression
Abstract
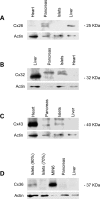
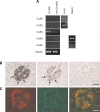
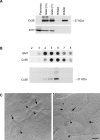
Previous studies have documented that the insulin-producing beta-cells of laboratory rodents are coupled by gap junction channels made solely of the connexin36 (Cx36) protein, and have shown that loss of this protein desynchronizes beta-cells, leading to secretory defects reminiscent of those observed in type 2 diabetes. Since human islets differ in several respects from those of laboratory rodents, we have now screened human pancreas, and islets isolated thereof, for expression of a variety of connexin genes, tested whether the cognate proteins form functional channels for islet cell exchanges, and assessed whether this expression changes with beta-cell function in islets of control and type 2 diabetics. Here, we show that (i) different connexin isoforms are differentially distributed in the exocrine and endocrine parts of the human pancreas; (ii) human islets express at the transcript level different connexin isoforms; (iii) the membrane of beta-cells harbors detectable levels of gap junctions made of Cx36; (iv) this protein is concentrated in lipid raft domains of the beta-cell membrane where it forms gap junctions; (v) Cx36 channels allow for the preferential exchange of cationic molecules between human beta-cells; (vi) the levels of Cx36 mRNA correlated with the expression of the insulin gene in the islets of both control and type 2 diabetics. The data show that Cx36 is a native protein of human pancreatic islets, which mediates the coupling of the insulin-producing beta-cells, and contributes to control beta-cell function by modulating gene expression.
Figures



References
-
- Saez J.C., Berthoud V.M., Branes M.C., Martinez A.D., Beyer E.C. Plasma membrane channels formed by connexins: their regulation and functions. Physiol. Rev. 2003;83:1359–1400. - PubMed
-
- Söhl G., Willecke K. Gap junctions and the connexin protein family. Cardiovasc. Res. 2004;62:228–232. - PubMed
-
- Michon L., Nlend Nlend R., Bavamian S., Bischoff L., Boucard N., Caille D., Cancela J., Charollais A., Charpantier E., Klee P., et al. Involvement of gap junctional communication in secretion. Biochim. Biophys. Acta. 2005;1719:82–101. - PubMed
-
- Bavamian S., Klee P., Britan A., Populaire C., Caille D., Cancela J., Charollais A., Meda P. Islet-cell-to-cell communication as basis for normal insulin secretion. Diabetes Obes. Metab. 2007;9(Suppl. 2):118–132. - PubMed
-
- Serre-Beinier V., Le Gurun S., Belluardo N., Trovato-Salinaro A., Charollais A., Haefliger J.A., Condorelli D.F., Meda P. Cx36 preferentially connects beta-cells within pancreatic islets. Diabetes. 2000;49:727–734. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

