Heart failure with preserved and reduced left ventricular ejection fraction in the antihypertensive and lipid-lowering treatment to prevent heart attack trial
- PMID: 19001024
- PMCID: PMC2775475
- DOI: 10.1161/CIRCULATIONAHA.107.762229
Heart failure with preserved and reduced left ventricular ejection fraction in the antihypertensive and lipid-lowering treatment to prevent heart attack trial
Abstract
Background: Heart failure (HF) developing in hypertensive patients may occur with preserved or reduced left ventricular ejection fraction (PEF [>or=50%] or REF [<50%]). In the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), 42 418 high-risk hypertensive patients were randomized to chlorthalidone, amlodipine, lisinopril, or doxazosin, providing an opportunity to compare these treatments with regard to occurrence of hospitalized HFPEF or HFREF.
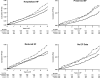
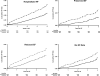
Methods and results: HF diagnostic criteria were prespecified in the ALLHAT protocol. EF estimated by contrast ventriculography, echocardiography, or radionuclide study was available in 910 of 1367 patients (66.6%) with hospitalized events meeting ALLHAT criteria. Cox regression models adjusted for baseline characteristics were used to examine treatment differences for HF (overall and by PEF and REF). HF case fatality rates were examined. Of those with EF data, 44.4% had HFPEF and 55.6% had HFREF. Chlorthalidone reduced the risk of HFPEF compared with amlodipine, lisinopril, or doxazosin; the hazard ratios were 0.69 (95% confidence interval [CI], 0.53 to 0.91; P=0.009), 0.74 (95% CI, 0.56 to 0.97; P=0.032), and 0.53 (95% CI, 0.38 to 0.73; P<0.001), respectively. Chlorthalidone reduced the risk of HFREF compared with amlodipine or doxazosin; the hazard ratios were 0.74 (95% CI, 0.59 to 0.94; P=0.013) and 0.61 (95% CI, 0.47 to 0.79; P<0.001), respectively. Chlorthalidone was similar to lisinopril with regard to incidence of HFREF (hazard ratio, 1.07; 95% CI, 0.82 to 1.40; P=0.596). After HF onset, death occurred in 29.2% of participants (chlorthalidone/amlodipine/lisinopril) with new-onset HFPEF versus 41.9% in those with HFREF (P<0.001; median follow-up, 1.74 years); and in the chlorthalidone/doxazosin comparison that was terminated early, 20.0% of HFPEF and 26.0% of HFREF patients died (P=0.185; median follow-up, 1.55 years).
Conclusions: In ALLHAT, with adjudicated outcomes, chlorthalidone significantly reduced the occurrence of new-onset hospitalized HFPEF and HFREF compared with amlodipine and doxazosin. Chlorthalidone also reduced the incidence of new-onset HFPEF compared with lisinopril. Among high-risk hypertensive men and women, HFPEF has a better prognosis than HFREF.
Trial registration: ClinicalTrials.gov NCT00000542.
Figures






Comment in
-
Hypertension, heart failure, and ejection fraction.Circulation. 2008 Nov 25;118(22):2223-4. doi: 10.1161/CIRCULATIONAHA.108.819318. Epub 2008 Nov 10. Circulation. 2008. PMID: 19001020 No abstract available.
-
Letter by barrios et Al regarding article, "heart failure with preserved and reduced left ventricular ejection fraction in the antihypertensive and lipid-lowering treatment to prevent heart attack trial".Circulation. 2009 Aug 4;120(5):e31; author reply e32. doi: 10.1161/CIRCULATIONAHA.108.844621. Circulation. 2009. PMID: 19652116 No abstract available.
References
-
- Davis BR, Cutler JA, Gordon DJ, Furberg CD, Wright JT, Jr, Cushman WC, Grimm RH, LaRosa J, Whelton PK, Perry HM, Alderman MH, Ford CE, Oparil S, Francis C, Proschan M, Pressel S, Black HR, Hawkins CM. Rationale and design for the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). ALLHAT Research Group. Am J Hypertens. 1996;9:342–360. - PubMed
-
- Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial l (ALLHAT). ALLHAT Collaborative Research Group. JAMA. 2000;283:1967–1975. - PubMed
-
- ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–2997. - PubMed
-
- Messerli FH. Doxazosin and congestive heart failure. J Am Coll Cardiol. 2001;38:1295–1296. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

