An energy supply network of nutrient absorption coordinated by calcium and T1R taste receptors in rat small intestine
- PMID: 19001049
- PMCID: PMC2670033
- DOI: 10.1113/jphysiol.2008.159616
An energy supply network of nutrient absorption coordinated by calcium and T1R taste receptors in rat small intestine
Abstract
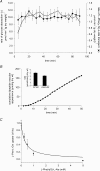
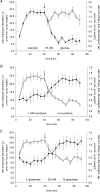
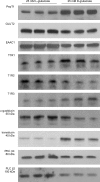
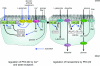
T1R taste receptors are present throughout the gastrointestinal tract. Glucose absorption comprises active absorption via SGLT1 and facilitated absorption via GLUT2 in the apical membrane. Trafficking of apical GLUT2 is rapidly up-regulated by glucose and artificial sweeteners, which act through T1R2 + T1R3/alpha-gustducin to activate PLC beta2 and PKC betaII. We therefore investigated whether non-sugar nutrients are regulated by taste receptors using perfused rat jejunum in vivo. Under different conditions, we observed a Ca(2+)-dependent reciprocal relationship between the H(+)/oligopeptide transporter PepT1 and apical GLUT2, reflecting the fact that trafficking of PepT1 and GLUT2 to the apical membrane is inhibited and activated by PKC betaII, respectively. Addition of L-glutamate or sucralose to a perfusate containing low glucose (20 mM) each activated PKC betaII and decreased apical PepT1 levels and absorption of the hydrolysis-resistant dipeptide L-Phe(PsiS)-L-Ala (1 mM), while increasing apical GLUT2 and glucose absorption within minutes. Switching perfusion from mannitol to glucose (75 mM) exerted similar effects. c-glutamate induced rapid GPCR internalization of T1R1, T1R3 and transducin, whereas sucralose internalized T1R2, T1R3 and alpha-gustducin. We conclude that L-glutamate acts via amino acid and glucose via sweet taste receptors to coordinate regulation of PepT1 and apical GLUT2 reciprocally through a common enterocytic pool of PKC betaII. These data suggest the existence of a wider Ca(2+) and taste receptor-coordinated transport network incorporating other nutrients and/or other stimuli capable of activating PKC betaII and additional transporters, such as the aspartate/glutamate transporter, EAAC1, whose level was doubled by L-glutamate. The network may control energy supply.
Figures



References
-
- Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000;100:693–702. - PubMed
-
- Bailey P. Drug delivery system. 2005. Appl. No. PCT/Gb2005/0001. Pub. No. WO2005/0067978.
-
- Bailey PD, Boyd CA, Collier ID, George JP, Kellett GL, Meredith D, Morgan KM, Pettecrew R, Price RA. Affinity prediction for substrates of the peptide transporter PepT1. Chem Commun (Camb) 2006:323–325. - PubMed
-
- Bailey PD, Boyd CA, Collier ID, Kellett GL, Meredith D, Morgan KM, Pettecrew R, Price RA. Probing dipeptide trans/cis stereochemistry using pH control of thiopeptide analogues, and application to the PepT1 transporter. Org Biomol Chem. 2005;3:4038–4039. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

