Characterization of Entamoeba histolytica intermediate subunit lectin-specific human monoclonal antibodies generated in transgenic mice expressing human immunoglobulin loci
- PMID: 19001071
- PMCID: PMC2612286
- DOI: 10.1128/IAI.01002-08
Characterization of Entamoeba histolytica intermediate subunit lectin-specific human monoclonal antibodies generated in transgenic mice expressing human immunoglobulin loci
Abstract
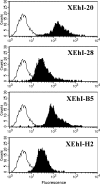
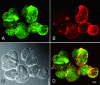
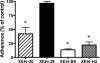
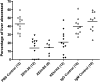
Four fully human monoclonal antibodies (MAbs) to Entamoeba histolytica intermediate subunit lectin (Igl) were prepared in XenoMouse mice, which are transgenic mice expressing human immunoglobulin loci. Examination of the reactivities of these MAbs to recombinant Igl1 and Igl2 of E. histolytica showed that XEhI-20 {immunoglobulin G2(kappa) [IgG2(kappa)]} and XEhI-28 [IgG2(kappa)] were specific to Igl1, XEhI-B5 [IgG2(kappa)] was specific to Igl2, and XEhI-H2 [IgM(kappa)] was reactive with both Igls. Gene analyses revealed that the V(H) and V(L) germ lines were VH3-48 and L2 for XEhI-20, VH3-21 and L2 for XEhI-28, VH3-33 and B3 for XEhI-B5, and VH4-4 and A19 for XEhI-H2, respectively. Flow cytometry analyses showed that the epitopes recognized by all of these MAbs were located on the surfaces of living trophozoites. Confocal microscopy demonstrated that most Igl1 and Igl2 proteins were colocalized on the surface and in the cytoplasm, but different localization patterns in intracellular vacuoles were also present. The preincubation of trophozoites with XEhI-20, XEhI-B5, and XEhI-H2 caused significant inhibition of the adherence of trophozoites to Chinese hamster ovary cells, whereas preincubation with XEhI-28 did not do so. XEhI-20, XEhI-B5, and XEhI-H2 were injected intraperitoneally into hamsters 24 h prior to intrahepatic challenge with E. histolytica trophozoites. One week later, the mean abscess size in groups injected with one of the three MAbs was significantly smaller than that in controls injected with polyclonal IgG or IgM isolated from healthy humans. These results demonstrate that human MAbs to Igls may be applicable for immunoprophylaxis of amebiasis.
Figures



References
-
- Abadi, J., J. Friedman, R. A. Mageed, R. Jefferis, M. C. Rodriguez-Barradas, and L. Pirofski. 1998. Human antibodies elicited by a pneumococcal vaccine express idiotypic determinants indicative of V(H)3 gene segment usage. J. Infect. Dis. 178707-716. - PubMed
-
- Andris, J. S., P. H. Ehrlich, L. Ostberg, and J. D. Capra. 1992. Probing the human antibody repertoire to exogenous antigens. Characterization of the H and L chain V region gene segments from anti-hepatitis B virus antibodies. J. Immunol. 1494053-4059. - PubMed
-
- Bekker, P. J., D. L. Holloway, A. S. Rasmussen, R. Murphy, S. W. Martin, P. T. Leese, G. B. Holmes, C. R. Dunstan, and A. M. DePaoli. 2004. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J. Bone Miner. Res. 191059-1066. - PubMed
-
- Bird, R. E., K. D. Hardman, J. W. Jacobson, S. Johnson, B. M. Kaufman, S. M. Lee, T. Lee, S. H. Pope, G. S. Riordan, and M. Whitlow. 1988. Single-chain antigen-binding proteins. Science 242423-426. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

