Antibody to the type 3 capsule facilitates immune adherence of pneumococci to erythrocytes and augments their transfer to macrophages
- PMID: 19001076
- PMCID: PMC2612250
- DOI: 10.1128/IAI.00892-08
Antibody to the type 3 capsule facilitates immune adherence of pneumococci to erythrocytes and augments their transfer to macrophages
Abstract
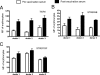
Streptococcus pneumoniae has been shown to bind to erythrocytes via a process called immune adherence. This adherence and the subsequent transfer of pneumococci from erythrocytes to macrophages are both dependent on complement C3 deposition onto the pneumococcal surface. The observation that anti-capsule antibody increases C3 deposition on the pneumococcal capsule indicated that anti-capsule antibody may also facilitate the clearance of pneumococci through immune adherence. Using pneumococcal strain WU2 (capsule type 3) and its nonencapsulated mutant JD908, we found that monoclonal antibody (MAb) to type 3 capsule increases complement C3, C1q, and C4 deposition on WU2 and enhanced the immune adherence of WU2 to erythrocytes. The MAb to type 3 capsule also enhanced the transfer of WU2 from erythrocytes to macrophages. Moreover, the transfer reaction was inhibited by preincubating macrophages with anti-CR3 or anti-Fc gammaRIII/II MAb, indicating that CR3 and Fc gammaRIII/II on macrophages mediate this process. The transfer reactions of JD908 (opsonized with complement) and WU2 (opsonized with complement plus MAb to type 3 capsule) were similarly inhibited by anti-CR3 MAb, but only the latter was inhibited by anti-Fc gammaRIII/II MAb. This finding indicates that although complement and the macrophage receptor CR3 are essential for the transfer reaction, if antibody is present it can further enhance the transfer reaction through a process dependent on Fc gammaRIII/II. Using pre- and postvaccination sera of people immunized with the 23-valent pneumococcal polysaccharide vaccine, we confirmed that human anti-capsule antibodies are also able to increase the immune adherence of pneumococci and their transfer to macrophages.
Figures

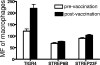
Similar articles
-
PspA and PspC minimize immune adherence and transfer of pneumococci from erythrocytes to macrophages through their effects on complement activation.Infect Immun. 2007 Dec;75(12):5877-85. doi: 10.1128/IAI.00839-07. Epub 2007 Oct 8. Infect Immun. 2007. PMID: 17923519 Free PMC article.
-
Complement receptor 1 expression on mouse erythrocytes mediates clearance of Streptococcus pneumoniae by immune adherence.Infect Immun. 2010 Jul;78(7):3129-35. doi: 10.1128/IAI.01263-09. Epub 2010 May 3. Infect Immun. 2010. PMID: 20439480 Free PMC article.
-
Effects of PspA and antibodies to PspA on activation and deposition of complement on the pneumococcal surface.Infect Immun. 2004 Jan;72(1):114-22. doi: 10.1128/IAI.72.1.114-122.2004. Infect Immun. 2004. PMID: 14688088 Free PMC article.
-
The absence of PspA or presence of antibody to PspA facilitates the complement-dependent phagocytosis of pneumococci in vitro.Clin Vaccine Immunol. 2012 Oct;19(10):1574-82. doi: 10.1128/CVI.00393-12. Epub 2012 Aug 1. Clin Vaccine Immunol. 2012. PMID: 22855389 Free PMC article.
-
Scientific memory from the early nineties; a common project with professors late János Gergely and Anna Erdei.Biol Futur. 2021 Mar;72(1):3-5. doi: 10.1007/s42977-020-00056-y. Epub 2021 Jan 10. Biol Futur. 2021. PMID: 34554495 Review.
Cited by
-
The efficacy of pneumococcal capsular polysaccharide-specific antibodies to serotype 3 Streptococcus pneumoniae requires macrophages.Vaccine. 2010 Nov 3;28(47):7542-50. doi: 10.1016/j.vaccine.2010.08.061. Epub 2010 Aug 26. Vaccine. 2010. PMID: 20800700 Free PMC article.
-
Impact of the glpQ2 gene on virulence in a Streptococcus pneumoniae serotype 19A sequence type 320 strain.Infect Immun. 2015 Feb;83(2):682-92. doi: 10.1128/IAI.02357-14. Epub 2014 Nov 24. Infect Immun. 2015. PMID: 25422269 Free PMC article.
-
Pleiotropic effects of cell wall amidase LytA on Streptococcus pneumoniae sensitivity to the host immune response.Infect Immun. 2015 Feb;83(2):591-603. doi: 10.1128/IAI.02811-14. Epub 2014 Nov 17. Infect Immun. 2015. PMID: 25404032 Free PMC article.
-
A serotype 3 pneumococcal capsular polysaccharide-specific monoclonal antibody requires Fcγ receptor III and macrophages to mediate protection against pneumococcal pneumonia in mice.Infect Immun. 2012 Apr;80(4):1314-22. doi: 10.1128/IAI.06081-11. Epub 2012 Jan 30. Infect Immun. 2012. PMID: 22290146 Free PMC article.
-
Aggregation of Streptococcus pneumoniae by a pneumococcal capsular polysaccharide-specific human monoclonal IgM correlates with antibody efficacy in vivo.Clin Vaccine Immunol. 2010 May;17(5):713-21. doi: 10.1128/CVI.00410-09. Epub 2010 Mar 3. Clin Vaccine Immunol. 2010. PMID: 20200186 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

