Characterization of Vibrio cholerae outer membrane vesicles as a candidate vaccine for cholera
- PMID: 19001078
- PMCID: PMC2612262
- DOI: 10.1128/IAI.01139-08
Characterization of Vibrio cholerae outer membrane vesicles as a candidate vaccine for cholera
Abstract
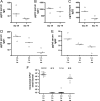
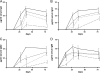
Outer membrane vesicles (OMVs) offer a new approach for an effective cholera vaccine. We recently demonstrated that immunization of female mice with OMVs induces a long-lasting immune response and results in protection of their neonatal offspring from Vibrio cholerae intestinal colonization. This study investigates the induced protective immunity observed after immunization with OMVs in more detail. Analysis of the stomach contents and sera of the neonates revealed significant amounts of anti-OMV immunoglobulins (Igs). Swapping of litters born to immunized and nonvaccinated control mice allowed us to distinguish between prenatal and neonatal uptakes of Igs. Transfer of Igs to neonates via milk was sufficient for complete protection of the neonates from colonization with V. cholerae, while prenatal transfer alone reduced colonization only. Detection of IgA and IgG1 in the fecal pellets of intranasally immunized adult mice indicates an induced immune response at the mucosal surface in the gastrointestinal tract, which is the site of colonization by V. cholerae. When a protocol with three intranasal immunizations 14 days apart was used, the OMVs proved to be efficacious at doses as low as 0.025 microg per immunization. This is almost equivalent to OMV concentrations found naturally in the supernatants of LB-grown cultures of V. cholerae. Heterologous expression of the periplasmic alkaline phosphatase (PhoA) of Escherichia coli resulted in the incorporation of PhoA into OMVs derived from V. cholerae. Intranasal immunization with OMVs loaded with PhoA induced a specific immune response against this heterologous antigen in mice. The detection of an immune response against this heterologously expressed protein is a promising step toward the potential use of OMVs as antigen delivery vehicles in vaccine design.
Figures


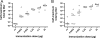
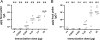

Similar articles
-
Mucosal immunization with Vibrio cholerae outer membrane vesicles provides maternal protection mediated by antilipopolysaccharide antibodies that inhibit bacterial motility.Infect Immun. 2010 Oct;78(10):4402-20. doi: 10.1128/IAI.00398-10. Epub 2010 Aug 2. Infect Immun. 2010. PMID: 20679439 Free PMC article.
-
Immunization with Vibrio cholerae outer membrane vesicles induces protective immunity in mice.Infect Immun. 2008 Oct;76(10):4554-63. doi: 10.1128/IAI.00532-08. Epub 2008 Aug 4. Infect Immun. 2008. PMID: 18678672 Free PMC article.
-
Immunization of mice with vibrio cholerae outer-membrane vesicles protects against hyperinfectious challenge and blocks transmission.J Infect Dis. 2012 Feb 1;205(3):412-21. doi: 10.1093/infdis/jir756. Epub 2011 Dec 5. J Infect Dis. 2012. PMID: 22147790 Free PMC article.
-
Exploiting cholera vaccines as a versatile antigen delivery platform.Biotechnol Lett. 2008 Apr;30(4):571-9. doi: 10.1007/s10529-007-9594-0. Epub 2007 Nov 16. Biotechnol Lett. 2008. PMID: 18008168 Free PMC article. Review.
-
Human Challenge Studies for Cholera.Curr Top Microbiol Immunol. 2024;445:177-188. doi: 10.1007/82_2022_258. Curr Top Microbiol Immunol. 2024. PMID: 35377003 Review.
Cited by
-
Immunization with Salmonella enterica serovar Typhimurium-derived outer membrane vesicles delivering the pneumococcal protein PspA confers protection against challenge with Streptococcus pneumoniae.Infect Immun. 2011 Feb;79(2):887-94. doi: 10.1128/IAI.00950-10. Epub 2010 Nov 29. Infect Immun. 2011. PMID: 21115718 Free PMC article.
-
Outer Membrane Vesicles of Vibrio cholerae Protect and Deliver Active Cholera Toxin to Host Cells via Porin-Dependent Uptake.mBio. 2021 Jun 29;12(3):e0053421. doi: 10.1128/mBio.00534-21. Epub 2021 May 26. mBio. 2021. PMID: 34076466 Free PMC article.
-
Immunization with cholera toxin B subunit induces high-level protection in the suckling mouse model of cholera.PLoS One. 2013;8(2):e57269. doi: 10.1371/journal.pone.0057269. Epub 2013 Feb 28. PLoS One. 2013. PMID: 23468950 Free PMC article.
-
Bacterial outer membrane vesicles in disease and preventive medicine.Semin Immunopathol. 2011 Sep;33(5):395-408. doi: 10.1007/s00281-010-0231-y. Epub 2010 Dec 12. Semin Immunopathol. 2011. PMID: 21153593 Review.
-
Chloroplast-derived vaccine antigens confer dual immunity against cholera and malaria by oral or injectable delivery.Plant Biotechnol J. 2010 Feb;8(2):223-42. doi: 10.1111/j.1467-7652.2009.00479.x. Epub 2009 Dec 28. Plant Biotechnol J. 2010. PMID: 20051036 Free PMC article.
References
-
- Ahouse, J. J., C. L. Hagerman, P. Mittal, D. J. Gilbert, N. G. Copeland, N. A. Jenkins, and N. E. Simister. 1993. Mouse MHC class I-like Fc receptor encoded outside the MHC. J. Immunol. 1516076-6088. - PubMed
-
- Alaniz, R. C., B. L. Deatherage, J. C. Lara, and B. T. Cookson. 2007. Membrane vesicles are immunogenic facsimiles of Salmonella typhimurium that potently activate dendritic cells, prime B and T cell responses, and stimulate protective immunity in vivo. J. Immunol. 1797692-7701. - PubMed
-
- Appleby, P., and D. Catty. 1983. Transmission of immunoglobulin to foetal and neonatal mice. J. Reprod. Immunol. 5203-213. - PubMed
-
- Bennish, M. L. 1994. Cholera: pathophysiology, clinical features, and treatment, p. 229-255. In K. I. Wachsmuth, P. A. Blake, and O. Olsik (ed.), Vibrio cholerae and cholera: molecular to global perspectives. ASM Press, Washington, DC.
-
- Bradford, A. K., A. B. Cheryl, and J. G. Wells. 1994. Isolation and identification of Vibrio cholerae O1 from fecal specimens, p. 3-25. In K. Wachsmut, P. A. Blake, and O. Olsvik (ed.), Vibrio cholerae and cholera: molecular to global perspectives. ASM Press, Washington, DC.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

