Contributions of F-BAR and SH2 domains of Fes protein tyrosine kinase for coupling to the FcepsilonRI pathway in mast cells
- PMID: 19001085
- PMCID: PMC2612524
- DOI: 10.1128/MCB.00904-08
Contributions of F-BAR and SH2 domains of Fes protein tyrosine kinase for coupling to the FcepsilonRI pathway in mast cells
Abstract
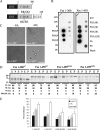
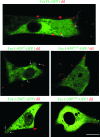
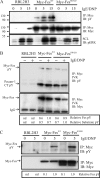
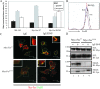
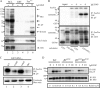
This study investigates the roles of Fer-CIP4 homology (FCH)-Bin/amphiphysin/Rvs (F-BAR) and SH2 domains of Fes protein tyrosine kinase in regulating its activation and signaling downstream of the high-affinity immunoglobulin G (IgE) receptor (FcepsilonRI) in mast cells. Homology modeling of the Fes F-BAR domain revealed conservation of some basic residues implicated in phosphoinositide binding (R113/K114). The Fes F-BAR can bind phosphoinositides and induce tubulation of liposomes in vitro. Mutation of R113/K114 to uncharged residues (RK/QQ) caused a significant reduction in phosphoinositide binding in vitro and a more diffuse cytoplasmic localization in transfected COS-7 cells. RBL-2H3 mast cells expressing full-length Fes carrying the RK/QQ mutation show defects in FcepsilonRI-induced Fes tyrosine phosphorylation and degranulation compared to cells expressing wild-type Fes. This correlated with reduced localization to Lyn kinase-containing membrane fractions for the RK/QQ mutant compared to wild-type Fes in mast cells. The Fes SH2 domain also contributes to Fes signaling in mast cells, via interactions with the phosphorylated FcepsilonRI beta chain and the actin regulatory protein HS1. We show that Fes phosphorylates C-terminal tyrosine residues in HS1 implicated in actin stabilization. Thus, coordinated actions of the F-BAR and SH2 domains of Fes allow for coupling to FcepsilonRI signaling and potential regulation the actin reorganization in mast cells.
Figures

References
-
- Abramson, J., and I. Pecht. 2007. Regulation of the mast cell response to the type 1 Fc epsilon receptor. Immunol. Rev. 217231-254. - PubMed
-
- Anderson, D. H., and P. M. Ismail. 1998. v-fps causes transformation by inducing tyrosine phosphorylation and activation of the PDGFβ receptor. Oncogene 162321-2331. - PubMed
-
- Aspenstrom, P., A. Fransson, and N. Richnau. 2006. Pombe Cdc15 homology proteins: regulators of membrane dynamics and the actin cytoskeleton. Trends Biochem. Sci. 31670-679. - PubMed
-
- Balla, T., and P. Varnai. 2002. Visualizing cellular phosphoinositide pools with GFP-fused protein-modules. Sci. STKE 2002PL3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
