Nucleus-specific and cell cycle-regulated degradation of mitogen-activated protein kinase scaffold protein Ste5 contributes to the control of signaling competence
- PMID: 19001089
- PMCID: PMC2612525
- DOI: 10.1128/MCB.01019-08
Nucleus-specific and cell cycle-regulated degradation of mitogen-activated protein kinase scaffold protein Ste5 contributes to the control of signaling competence
Abstract
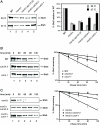
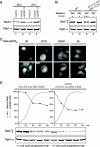
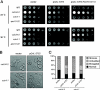
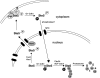
Saccharomyces cerevisiae cells are capable of responding to mating pheromone only prior to their exit from the G(1) phase of the cell cycle. Ste5 scaffold protein is essential for pheromone response because it couples pheromone receptor stimulation to activation of the appropriate mitogen-activated protein kinase (MAPK) cascade. In naïve cells, Ste5 resides primarily in the nucleus. Upon pheromone treatment, Ste5 is rapidly exported from the nucleus and accumulates at the tip of the mating projection via its association with multiple plasma membrane-localized molecules. We found that concomitant with its nuclear export, the rate of Ste5 turnover is markedly reduced. Preventing nuclear export destabilized Ste5, whereas preventing nuclear entry stabilized Ste5, indicating that Ste5 degradation occurs mainly in the nucleus. This degradation is dependent on ubiquitin and the proteasome. We show that Ste5 ubiquitinylation is mediated by the SCF(Cdc4) ubiquitin ligase and requires phosphorylation by the G(1) cyclin-dependent protein kinase (cdk1). The inability to efficiently degrade Ste5 resulted in pathway activation and cell cycle arrest in the absence of pheromone. These findings reveal that maintenance of this MAPK scaffold at an appropriately low level depends on its compartment-specific and cell cycle-dependent degradation. Overall, this mechanism provides a novel means for helping to prevent inadvertent stimulus-independent activation of a response and for restricting and maximizing the signaling competence of the cell to a specific cell cycle stage, which likely works hand in hand with the demonstrated role that G(1) Cdk1-dependent phosphorylation of Ste5 has in preventing its association with the plasma membrane.
Figures

References
-
- Bai, C., P. Sen, K. Hofmann, L. Ma, M. Goebl, J. W. Harper, and S. J. Elledge. 1996. SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell 86263-274. - PubMed
-
- Bailer, S. M., C. Balduf, J. Katahira, A. Podtelejnikov, C. Rollenhagen, M. Mann, N. Pante, and E. Hurt. 2000. Nup116p associates with the Nup82p-Nsp1p-Nup159p nucleoporin complex. J. Biol. Chem. 27523540-23548. - PubMed
-
- Bashor, C. J., N. C. Helman, S. Yan, and W. A. Lim. 2008. Using engineered scaffold interactions to reshape MAP kinase pathway signaling dynamics. Science 3191539-1543. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
