Deletion of Shp2 tyrosine phosphatase in muscle leads to dilated cardiomyopathy, insulin resistance, and premature death
- PMID: 19001090
- PMCID: PMC2612510
- DOI: 10.1128/MCB.01661-08
Deletion of Shp2 tyrosine phosphatase in muscle leads to dilated cardiomyopathy, insulin resistance, and premature death
Abstract
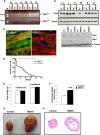
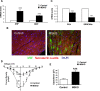
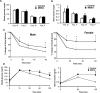
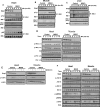
The intracellular signaling mechanisms underlying the pathogenesis of cardiac diseases are not fully understood. We report here that selective deletion of Shp2, an SH2-containing cytoplasmic tyrosine phosphatase, in striated muscle results in severe dilated cardiomyopathy in mice, leading to heart failure and premature mortality. Development of cardiomyopathy in this mouse model is coupled with insulin resistance, glucose intolerance, and impaired glucose uptake in striated muscle cells. Shp2 deficiency leads to upregulation of leukemia inhibitory factor-stimulated phosphatidylinositol 3-kinase/Akt, Erk5, and Stat3 pathways in cardiomyocytes. Insulin resistance and impaired glucose uptake in Shp2-deficient mice are at least in part due to impaired protein kinase C-zeta/lambda and AMP-kinase activities in striated muscle. Thus, we have generated a mouse line modeling human patients suffering from cardiomyopathy and insulin resistance. This study reinforces a concept that a compound disease with multiple cardiovascular and metabolic disturbances can be caused by a defect in a single molecule such as Shp2, which modulates multiple signaling pathways initiated by cytokines and hormones.
Figures



References
-
- Akazawa, H., and I. Komuro. 2003. Roles of cardiac transcription factors in cardiac hypertrophy. Circ. Res. 921079-1088. - PubMed
-
- Bruning, J. C., M. D. Michael, J. N. Winnay, T. Hayashi, D. Horsch, D. Accili, L. J. Goodyear, and C. R. Kahn. 1998. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Mol. Cell 2559-569. - PubMed
-
- Camper-Kirby, D., S. Welch, A. Walker, I. Shiraishi, K. D. Setchell, E. Schaefer, J. Kajstura, P. Anversa, and M. A. Sussman. 2001. Myocardial Akt activation and gender: increased nuclear activity in females versus males. Circ. Res. 881020-1027. - PubMed
-
- Carroll, R., A. N. Carley, J. R. Dyck, and D. L. Severson. 2005. Metabolic effects of insulin on cardiomyocytes from control and diabetic db/db mouse hearts. Am. J. Physiol. Endocrinol. Metab. 288E900-E906. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
