AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity
- PMID: 19001126
- PMCID: PMC2582894
- DOI: 10.1083/jcb.200806112
AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity
Abstract
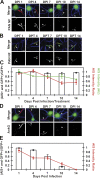
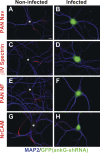
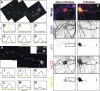
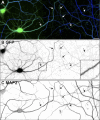
The axon initial segment (AIS) functions as both a physiological and physical bridge between somatodendritic and axonal domains. Given its unique molecular composition, location, and physiology, the AIS is thought to maintain neuronal polarity. To identify the molecular basis of this AIS property, we used adenovirus-mediated RNA interference to silence AIS protein expression in polarized neurons. Some AIS proteins are remarkably stable with half-lives of at least 2 wk. However, silencing the expression of the cytoskeletal scaffold ankyrinG (ankG) dismantles the AIS and causes axons to acquire the molecular characteristics of dendrites. Both cytoplasmic- and membrane-associated proteins, which are normally restricted to somatodendritic domains, redistribute into the former axon. Furthermore, spines and postsynaptic densities of excitatory synapses assemble on former axons. Our results demonstrate that the loss of ankG causes axons to acquire the molecular characteristics of dendrites; thus, ankG is required for the maintenance of neuronal polarity and molecular organization of the AIS.
Figures
References
-
- Arimura, N., and K. Kaibuchi. 2007. Neuronal polarity: from extracellular signals to intracellular mechanisms. Nat. Rev. Neurosci. 8:194–205. - PubMed
-
- Garrido, J.J., P. Giraud, E. Carlier, F. Fernandes, A. Moussif, M.P. Fache, D. Debanne, and B. Dargent. 2003. A targeting motif involved in sodium channel clustering at the axonal initial segment. Science. 300:2091–2094. - PubMed
-
- Gomis-Ruth, S., C.J. Wierenga, and F. Bradke. 2008. Plasticity of polarization: changing dendrites into axons in neurons integrated in neuronal circuits. Curr. Biol. 18:992–1000. - PubMed
-
- Grieco, T.M., J.D. Malhotra, C. Chen, L.L. Isom, and I.M. Raman. 2005. Open-channel block by the cytoplasmic tail of sodium channel β4 as a mechanism for resurgent sodium current. Neuron. 45:233–244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

