In vivo switch to IL-10-secreting T regulatory cells in high dose allergen exposure
- PMID: 19001136
- PMCID: PMC2585856
- DOI: 10.1084/jem.20080193
In vivo switch to IL-10-secreting T regulatory cells in high dose allergen exposure
Abstract
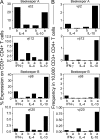
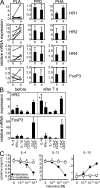
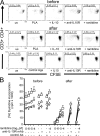
High dose bee venom exposure in beekeepers by natural bee stings represents a model to understand mechanisms of T cell tolerance to allergens in healthy individuals. Continuous exposure of nonallergic beekeepers to high doses of bee venom antigens induces diminished T cell-related cutaneous late-phase swelling to bee stings in parallel with suppressed allergen-specific T cell proliferation and T helper type 1 (Th1) and Th2 cytokine secretion. After multiple bee stings, venom antigen-specific Th1 and Th2 cells show a switch toward interleukin (IL) 10-secreting type 1 T regulatory (Tr1) cells. T cell regulation continues as long as antigen exposure persists and returns to initial levels within 2 to 3 mo after bee stings. Histamine receptor 2 up-regulated on specific Th2 cells displays a dual effect by directly suppressing allergen-stimulated T cells and increasing IL-10 production. In addition, cytotoxic T lymphocyte-associated antigen 4 and programmed death 1 play roles in allergen-specific T cell suppression. In contrast to its role in mucosal allergen tolerance, transforming growth factor beta does not seem to be an essential player in skin-related allergen tolerance. Thus, rapid switch and expansion of IL-10-producing Tr1 cells and the use of multiple suppressive factors represent essential mechanisms in immune tolerance to a high dose of allergens in nonallergic individuals.
Figures


Similar articles
-
High-dose bee venom exposure induces similar tolerogenic B-cell responses in allergic patients and healthy beekeepers.Allergy. 2017 Mar;72(3):407-415. doi: 10.1111/all.12966. Epub 2016 Jul 20. Allergy. 2017. PMID: 27341567
-
Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: the role of T regulatory cells.Immunology. 2006 Apr;117(4):433-42. doi: 10.1111/j.1365-2567.2006.02321.x. Immunology. 2006. PMID: 16556256 Free PMC article. Review.
-
Helios-Negative Regulatory T Cells as a Key Factor of Immune Tolerance in Nonallergic Beekeepers.J Investig Allergol Clin Immunol. 2022 Dec 15;32(6):451-459. doi: 10.18176/jiaci.0722. Epub 2022 Jul 2. J Investig Allergol Clin Immunol. 2022. PMID: 34213416
-
Insect venom immunotherapy induces interleukin-10 production and a Th2-to-Th1 shift, and changes surface marker expression in venom-allergic subjects.Eur J Immunol. 1997 May;27(5):1131-9. doi: 10.1002/eji.1830270513. Eur J Immunol. 1997. PMID: 9174602
-
T-cell tolerance to inhaled allergens: mechanisms and therapeutic approaches.Expert Opin Biol Ther. 2008 Jun;8(6):769-77. doi: 10.1517/14712598.8.6.769. Expert Opin Biol Ther. 2008. PMID: 18476788 Review.
Cited by
-
Mechanisms of allergen-specific immunotherapy and immune tolerance to allergens.World Allergy Organ J. 2015 May 14;8(1):17. doi: 10.1186/s40413-015-0063-2. eCollection 2015. World Allergy Organ J. 2015. PMID: 26023323 Free PMC article.
-
IFNγ/IL-10 co-producing cells dominate the CD4 response to malaria in highly exposed children.PLoS Pathog. 2014 Jan;10(1):e1003864. doi: 10.1371/journal.ppat.1003864. Epub 2014 Jan 9. PLoS Pathog. 2014. PMID: 24415936 Free PMC article. Clinical Trial.
-
Conversion of Anergic T Cells Into Foxp3- IL-10+ Regulatory T Cells by a Second Antigen Stimulus In Vivo.Front Immunol. 2021 Jun 25;12:704578. doi: 10.3389/fimmu.2021.704578. eCollection 2021. Front Immunol. 2021. PMID: 34249012 Free PMC article.
-
Treg-specific deletion of the phosphatase SHP-1 impairs control of inflammation in vivo.Front Immunol. 2023 Mar 16;14:1139326. doi: 10.3389/fimmu.2023.1139326. eCollection 2023. Front Immunol. 2023. PMID: 37006301 Free PMC article.
-
Tracking antigen-specific T-cells during clinical tolerance induction in humans.PLoS One. 2010 Jun 9;5(6):e11028. doi: 10.1371/journal.pone.0011028. PLoS One. 2010. PMID: 20543955 Free PMC article.
References
-
- Mosmann, T.R., H. Cherwinski, M.W. Bond, M.A. Giedlin, and R.L. Coffman. 1986. Two types of murine helper T cell clones. 1. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136:2348–2357. - PubMed
-
- Sakaguchi, S., and F. Powrie. 2007. Emerging challenges in regulatory T cell function and biology. Science. 317:627–629. - PubMed
-
- Roncarolo, M.G., and M. Battaglia. 2007. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat. Rev. Immunol. 7:585–598. - PubMed
-
- Hill, J.A., C. Benoist, and D. Mathis. 2007. Treg cells: guardians for life. Nat. Immunol. 8:124–125. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

