Intralymphatic allergen administration renders specific immunotherapy faster and safer: a randomized controlled trial
- PMID: 19001265
- PMCID: PMC2582048
- DOI: 10.1073/pnas.0803725105
Intralymphatic allergen administration renders specific immunotherapy faster and safer: a randomized controlled trial
Abstract
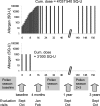
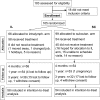
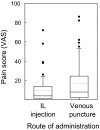
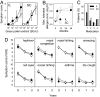
The only causative treatment for IgE-mediated allergies is allergen-specific immunotherapy. However, fewer than 5% of allergy patients receive immunotherapy because of its long duration and risk of allergic side effects. We aimed at enhancing s.c. immunotherapy by direct administration of allergen into s.c. lymph nodes. The objective was to evaluate safety and efficacy compared with conventional s.c. immunotherapy. In a monocentric open-label trial, 165 patients with grass pollen-induced rhinoconjunctivitis were randomized to receive either 54 s.c. injections with pollen extract over 3 years [cumulative allergen dose 4,031,540 standardized quality units (SQ-U)] or 3 intralymphatic injections over 2 months (cumulative allergen dose 3,000 SQ-U). Patients were evaluated after 4 months, 1 year, and 3 years by nasal provocation, skin prick testing, IgE measurements, and symptom scores. Three low-dose intralymphatic allergen administrations increased tolerance to nasal provocation with pollen already within 4 months (P < 0.001). Tolerance was long lasting and equivalent to that achievable after standard s.c. immunotherapy (P = 0.291 after 3 years). Intralymphatic immunotherapy ameliorated hay fever symptoms (P < 0.001), reduced skin prick test reactivity (P < 0.001), decreased specific serum IgE (P < 0.001), caused fewer adverse events than s.c. immunotherapy (P = 0.001), enhanced compliance (P < 0.001), and was less painful than venous puncture (P = 0.018). In conclusion, intralymphatic allergen administration enhanced safety and efficacy of immunotherapy and reduced treatment time from 3 years to 8 weeks.
Trial registration: ClinicalTrials.gov NCT00470457.
Conflict of interest statement
Conflict of interest statement: The corresponding author, T.M.K., is named as the inventor on a patent on intralymphatic immunotherapy. The patent is owned by the University of Zurich.
Figures

References
-
- Arbes SJ, Jr, Gergen PJ, Elliott L, Zeldin DC. Prevalences of positive skin test responses to 10 common allergens in the US population: Results from the third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2005;116:377–383. - PubMed
-
- Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: Therapeutic vaccines for allergic diseases. A WHO position paper. J Allergy Clin Immunol. 1998;102:558–562. - PubMed
-
- Lockey RF. “ARIA”: Global guidelines and new forms of allergen immunotherapy. J Allergy Clin Immunol. 2001;108:497–499. - PubMed
-
- Durham SR, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468–475. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

