Roles of PCNA-binding and ubiquitin-binding domains in human DNA polymerase eta in translesion DNA synthesis
- PMID: 19001268
- PMCID: PMC2584706
- DOI: 10.1073/pnas.0809844105
Roles of PCNA-binding and ubiquitin-binding domains in human DNA polymerase eta in translesion DNA synthesis
Abstract
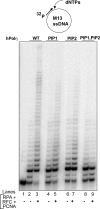
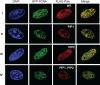
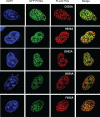
Treatment of yeast and human cells with DNA-damaging agents elicits Rad6-Rad18-mediated monoubiquitination of proliferating cell nuclear antigen (PCNA) at its Lys-164 residue [ubiquitin (Ub)-PCNA], and this PCNA modification is indispensable for promoting the access of translesion synthesis (TLS) polymerases (Pols) to PCNA. However, the means by which K164-linked Ub modulates the proficiency of TLS Pols to bind PCNA and take over synthesis from the replicative Pol has remained unclear. One model that has gained considerable credence is that the TLS Pols bind PCNA at 2 sites, to the interdomain connector loop via their PCNA-interacting protein (PIP) domain and to the K164-linked Ub moiety via their Ub-binding domain (UBD). Specifically, this model postulates that the UBD-mediated binding of TLS Pols to the Ub moiety on PCNA is necessary for TLS. To test the validity of this model, we examine the contributions that the PIP and Ub-binding zinc finger (UBZ) domains of human Poleta make to its functional interaction with PCNA, its colocalization with PCNA in replication foci, and its role in TLS in vivo. We conclude from these studies that the binding to PCNA via its PIP domain is a prerequisite for Poleta's ability to function in TLS in human cells and that the direct binding of the Ub moiety on PCNA via its UBZ domain is not required. We discuss the possible role of the Ub moiety on PCNA in TLS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

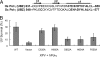

Comment in
-
Ubiquitin-binding motif of human DNA polymerase eta is required for correct localization.Proc Natl Acad Sci U S A. 2009 Feb 24;106(8):E20; author reply E21. doi: 10.1073/pnas.0812744106. Proc Natl Acad Sci U S A. 2009. PMID: 19240217 Free PMC article. No abstract available.
References
-
- Prakash S, Johnson RE, Prakash L. Eukaryotic translesion synthesis DNA polymerases: Specificity of structure and function. Annu Rev Biochem. 2005;74:317–353. - PubMed
-
- Johnson RE, Prakash S, Prakash L. Efficient bypass of a thymine–thymine dimer by yeast DNA polymerase, Polη. Science. 1999;283:1001–1004. - PubMed
-
- Johnson RE, Kondratick CM, Prakash S, Prakash L. hRAD30 mutations in the variant form of xeroderma pigmentosum. Science. 1999;285:263–265. - PubMed
-
- Lone S, et al. Human DNA polymerase κ encircles DNA: Implications for mismatch extension and lesion bypass. Mol Cell. 2007;25:601–614. - PubMed
-
- Masutani C, et al. The XPV (xeroderma pigmentosum variant) gene encodes human DNA polymerase η. Nature. 1999;399:700–704. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

