Domain compliance and elastic power transmission in rotary F(O)F(1)-ATPase
- PMID: 19001275
- PMCID: PMC2584700
- DOI: 10.1073/pnas.0807683105
Domain compliance and elastic power transmission in rotary F(O)F(1)-ATPase
Abstract
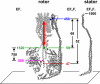
The 2 nanomotors of rotary ATP synthase, ionmotive F(O) and chemically active F(1), are mechanically coupled by a central rotor and an eccentric bearing. Both motors rotate, with 3 steps in F(1) and 10-15 in F(O). Simulation by statistical mechanics has revealed that an elastic power transmission is required for a high rate of coupled turnover. Here, we investigate the distribution in the F(O)F(1) structure of compliant and stiff domains. The compliance of certain domains was restricted by engineered disulfide bridges between rotor and stator, and the torsional stiffness (kappa) of unrestricted domains was determined by analyzing their thermal rotary fluctuations. A fluorescent magnetic bead was attached to single molecules of F(1) and a fluorescent actin filament to F(O)F(1), respectively. They served to probe first the functional rotation and, after formation of the given disulfide bridge, the stochastic rotational motion. Most parts of the enzyme, in particular the central shaft in F(1), and the long eccentric bearing were rather stiff (torsional stiffness kappa > 750 pNnm). One domain of the rotor, namely where the globular portions of subunits gamma and epsilon of F(1) contact the c-ring of F(O), was more compliant (kappa congruent with 68 pNnm). This elastic buffer smoothes the cooperation of the 2 stepping motors. It is located were needed, between the 2 sites where the power strokes in F(O) and F(1) are generated and consumed.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
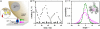



Similar articles
-
Two rotary motors in F-ATP synthase are elastically coupled by a flexible rotor and a stiff stator stalk.Proc Natl Acad Sci U S A. 2011 Mar 8;108(10):3924-9. doi: 10.1073/pnas.1011581108. Epub 2011 Feb 22. Proc Natl Acad Sci U S A. 2011. PMID: 21368147 Free PMC article.
-
Rotor/Stator interactions of the epsilon subunit in Escherichia coli ATP synthase and implications for enzyme regulation.J Biol Chem. 2004 Aug 20;279(34):35616-21. doi: 10.1074/jbc.M405012200. Epub 2004 Jun 15. J Biol Chem. 2004. PMID: 15199054
-
F-ATPase: forced full rotation of the rotor despite covalent cross-link with the stator.J Biol Chem. 2001 Nov 9;276(45):42287-92. doi: 10.1074/jbc.M106884200. Epub 2001 Aug 31. J Biol Chem. 2001. PMID: 11533065
-
Structures and interactions of proteins involved in the coupling function of the protonmotive F(o)F(1)-ATP synthase.Curr Protein Pept Sci. 2002 Aug;3(4):451-60. doi: 10.2174/1389203023380558. Curr Protein Pept Sci. 2002. PMID: 12370007 Review.
-
Inter-subunit rotation and elastic power transmission in F0F1-ATPase.FEBS Lett. 2001 Aug 31;504(3):152-60. doi: 10.1016/s0014-5793(01)02745-4. FEBS Lett. 2001. PMID: 11532447 Review.
Cited by
-
F1FO ATP synthase molecular motor mechanisms.Front Microbiol. 2022 Aug 23;13:965620. doi: 10.3389/fmicb.2022.965620. eCollection 2022. Front Microbiol. 2022. PMID: 36081786 Free PMC article. Review.
-
Structural Asymmetry and Kinetic Limping of Single Rotary F-ATP Synthases.Molecules. 2019 Jan 30;24(3):504. doi: 10.3390/molecules24030504. Molecules. 2019. PMID: 30704145 Free PMC article. Review.
-
Torque generation and elastic power transmission in the rotary F(O)F(1)-ATPase.Nature. 2009 May 21;459(7245):364-70. doi: 10.1038/nature08145. Nature. 2009. PMID: 19458712 Review.
-
Characterization of the relationship between ADP- and epsilon-induced inhibition in cyanobacterial F1-ATPase.J Biol Chem. 2011 Apr 15;286(15):13423-9. doi: 10.1074/jbc.M110.155986. Epub 2011 Feb 23. J Biol Chem. 2011. PMID: 21345803 Free PMC article.
-
FliI6-FliJ molecular motor assists with unfolding in the type III secretion export apparatus.Sci Rep. 2020 Apr 28;10(1):7127. doi: 10.1038/s41598-020-63330-y. Sci Rep. 2020. PMID: 32346005 Free PMC article.
References
-
- Junge W, Lill H, Engelbrecht S. ATP synthase: An electrochemical transducer with rotatory mechanics. Trends Biochem Sci. 1997;22:420–423. - PubMed
-
- Wang HY, Oster G. Energy transduction in the F1 motor of ATP synthase. Nature. 1998;396:279–282. - PubMed
-
- Junge W, et al. Inter-subunit rotation and elastic power transmission in FoF1-ATPase. FEBS Lett. 2001;504:152–160. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

